Aqueous ionic liquids and deep eutectic solvents for cellulosic biomass pretreatment and saccharification†
Shuqian Xiaa,
Gary A. Bakerb,
Hao Lia,
Sudhir Ravulab and
Hua Zhao*c
aSchool of Chemical Engineering & Technology, Tianjin University, Tianjin 300072, China
bDepartment of Chemistry, University of Missouri-Columbia, Columbia, MO 65211, USA
cDepartment of Chemistry and Forensic Science, Savannah State University, Savannah, GA 31404, USA. E-mail: huazhao98@gmail.com; zhaoh@savannahstate.edu; Tel: +1-912-358-4448
First published on 19th December 2013
Abstract
Ionic liquids (ILs) have proven effective solvents for pretreating lignocellulose, leading to the fast saccharification of cellulose and hemicellulose. However, the high cost of most ILs remains a major barrier to commercializing this recent approach at a practical scale. As a strategic detour, aqueous solutions of ILs are also being explored as less costly alternatives to neat ILs for cellulose pretreatment. However, limited studies on a few select IL systems are known and there remains no systematic survey of various ILs, eluding an in-depth understanding of pretreatment mechanisms afforded by aqueous IL systems. As a step toward filling this gap, this study presents results for Avicel cellulose pretreatment by neat and aqueous solutions (1.0 and 2.0 M) of 20 different ILs and three deep eutectic solvents, correlating enzymatic hydrolysis rates of pretreated cellulose with various IL properties such as hydrogen-bond basicity, polarity, Hofmeister ranking, and hydrophobicity. The pretreatment efficiencies of neat ILs may be loosely correlated to the hydrogen-bond basicity of the constituent anion and IL polarity; however, the pretreatment efficacies for aqueous ILs are more complicated and cannot be simply related to any single IL property. Several aqueous IL systems have been identified as effective alternatives to neat ILs in lignocellulose pretreatment. In particular, this study reveals that aqueous solutions of 1-butyl-3-methylimidazolium methanesulfonate ([BMIM][MeSO3]) are effective for pretreating switchgrass (Panicum virgatum), resulting in fast saccharification of both cellulose and hemicellulose. An integrated analysis afforded by X-ray diffraction, scanning electron microscopy, thermogravimetric analysis and cellulase adsorption isotherm of lignocellulose samples is further used to deliver a more complete view of the structural changes attending aqueous IL pretreatment.
1 Introduction
Ionic liquids (ILs) are unconventional, essentially non-volatile ionic organic solvents with melting points generally below 100 °C. Some ILs are capable of dissolving cellulosic biomass and have thus become the subject of intensive study as alternative media for lignocellulose pretreatment toward bioethanol production.1–3 Although IL pretreatment often leads to an accelerated saccharification of cellulosic biomass, this method has encountered several obstacles associated with ILs such as their high cost, energy intensive recycling, and environmental concerns over their toxicity and biodegradability.4,5 It is worth noting that the high IL cost is at least in part due to the pervasive use of the currently expensive imidazolium cations in designing cellulose-dissolving ILs.To address the high cost issue of ILs, a number of different approaches have been considered in addition to the recycling and reuse of ILs.6–8 The first approach entails the synthesis of some novel but less expensive ILs for cellulose dissolution. Along these lines of inquiry, our group has recently prepared relatively inexpensive and low-viscosity ether-functionalized alkylammonium and piperidinium ILs bearing acetate anions that are able to dissolve ∼10 wt% cellulose.9,10 Other groups have prepared cellulose-dissolving ILs containing amino acid-derived anions: (a) [EMIM][glycinate] reportedly afforded the complete dissolution of bamboo biomass,11 and (b) N,N-diethyl-N-(2-methoxyethyl)-N-methylammonium ILs containing amino acid anions like alaninate were able to dissolve 5–12 wt% cellulose at 100 °C.12 King et al.13 prepared novel guanidine-based ‘distillable’ acid–base conjugate ILs for dissolving microcrystalline cellulose (up to 5–10 wt%) at 100 °C. Zhang et al.14 developed a new CO2-triggered switchable system by mixing DMSO, non-ionic bases, and CO2. This switchable solvent platform was able to dissolve up to 15 wt% microcrystalline cellulose; the regenerated cellulose displayed a reduced crystallinity and a fast hydrolysis rate.
A second strategy for improving the economics of IL-based cellulose pretreatment is substituting neat ILs with ionic or eutectic mixtures. For example, the Jérôme group15 claimed that inexpensive ionic mixtures of cholinium acetate with 5–15 wt% of tributylmethylammonium chloride could dissolve 2–6 wt% microcrystalline cellulose (i.e., Avicel PH 105) at 110 °C. Rinaldi16 reported that amide-related solvents (such as 1,3-dimethyl-2-imidazolidinone and DMSO) containing a small fraction of [EMIM][OAc] or [BMIM]Cl (at molar fractions below 0.3, for instance) could instantaneously dissolve 10 wt% cellulose at 100 °C. In another example, the Francisco group17 designed biorenewable deep eutectic solvents (DESs) based on betaine, choline, and amino acids coupled with lactic or malic acid for dissolving large amounts (up to 12 to 15 wt%) of lignin (despite the poor solubility of cellulose), and further demonstrated the utility of their DESs in the delignification of wheat straw.
A third approach used to minimize the cost associated with using ILs to treat cellulosic materials involves the addition of compatible organic co-solvents. Unlike solvents such as alcohols, the co-solvents selected for this application are those that do not precipitate cellulose from the IL solution, at least within the chosen solvent composition range. In recent work, the Heinze group18 systematically examined the impact of 18 solvents and three binary solvent mixtures on cellulose solutions in IL and indicated that ideal co-solvents (such as DMSO, DMF and dichloromethane) typically have solvatochromic polarity ENT values above 0.3, very low ‘acidity’ (α < 0.5), and relatively high ‘basicity’ (β ≥ 0.4). The use of these co-solvents not only forms homogeneous cellulose solutions in ILs, but also considerably reduces the cost and viscosity of the resulting IL-based system. Tian et al.19 examined the enzymatic digestibility of microcrystalline cellulose pretreated by organic electrolyte solutions (mixtures of 1-allyl-3-methylimidazolium chloride ([AMIM]Cl) with DMSO) and suggested that the hydrolysis yield and rate increased with the mole fraction of [AMIM]Cl in the mixture; after cellulose pretreatment by an organic electrolyte solution containing 0.7 mole fraction of [AMIM]Cl, they achieved 54.1% glucose yield, which was 7.2 times higher than that of untreated cellulose.
Yet a fourth approach concerns pretreating cellulose with aqueous solutions of ILs (i.e., as opposed to neat ILs). For example, the Mazza group20,21 achieved an optimum fermentable sugar recovery of 71.4% from wheat straw when using an aqueous solution of 49.5 wt% [EMIM][OAc] at 158 °C for 3.6 h. Brandt et al.22 obtained ca. 90% glucose yields from cellulosic biomass pretreated by aqueous solutions of 80 vol.% [BMIM][MeSO3], [BMIM][MeSO4], or [BMIM][HSO4] at 120 °C for 22 h, but observed lower saccharification yields after pretreatment with 80 vol.% acetate-based ILs. The Zhang group23 pretreated sugarcane bagasse at 130 °C for 30 min by aqueous [BMIM]Cl solution containing 1.2% HCl, leading to a glucan recovery of >90% and digestibility of 94–100% after 72 h of enzymatic hydrolysis; however, the acidic condition resulted in the loss of most xylan. Although these aqueous ILs appear promising alternatives to neat ILs for lignocellulose pretreatment, there currently exists no study screening a wide spectrum of ILs. In addition, although the cellulose dissolution capability of neat ILs is often associated with the hydrogen-bond basicity of their constituent anions,9,24 it is not currently understood how aqueous ILs impact biomass pretreatment, as cellulose is insoluble in known aqueous IL systems.
Aiming at an in-depth understanding of cellulose pretreatment by aqueous ILs, we set out to determine and compare saccharification rates for cellulose pretreated with 20 different ILs and three cholinium-based DESs, neat and at 1.0 and 2.0 M concentrations in water. In order to better understand the pretreatment process, we also examined the cellulosic structure by X-ray diffraction (XRD), scanning electron microscopy (SEM), and thermogravimetric analysis (TGA), as well as attempting to correlate the enzymatic hydrolysis rate with the physical properties of the ILs. Finally, we also tested the saccharification efficiency for switchgrass pretreated with neat and aqueous ILs. The mechanistic insights offered by our systematic study bring into focus a clearer picture of what future role aqueous ILs might play in lignocellulose pretreatment and saccharification.
2 Experimental
2.1 Materials
A number of ILs were acquired from commercial sources, although several were prepared in house (see Table S1 in (ESI†) for details). The following chemicals and enzymes were purchased from Sigma-Aldrich (St. Louis, MO): Avicel PH-101 cellulose (microcrystalline cellulose (MCC), particle size 50 μm, DP 225, produced by FMC Corp.), cellulase from Trichoderma reesei (product # 22173, lot # 1086305, E.C. #3.2.1.4, slightly brown powder, 6.7 U mg−1 or 0.5 FPU mg−1) and glucose (HK) assay kit. β-Glucosidase from sweet almonds was obtained from MP biomedicals (product # 100348, lot #R28034, 3811 U mg−1‡). Switchgrass was a kind gift from Dr H. M. O'Neill (Oak Ridge National Laboratory); it was grown, harvested, dried, and then knife-milled to an average particle size of 0.5 mm. D-Xylose assay kit (catalog # K-XYLOSE) was obtained from the Megazyme International Ireland Ltd. (Bray, Co. Wicklow, Ireland).2.2 Pretreatment of Avicel PH-101 and switchgrass by IL solutions
Most attempts at IL pretreatment of cellulosic biomass can be subsumed under two broad categories: regeneration pretreatment and suspension pretreatment. Both avenues were involved in the course of the current work. (1) Regeneration pretreatment. When employing neat cellulose-dissolving ILs, 2.0 mL of an IL within a glass test tube was immersed in an oil bath held at 110 °C. Samples of cellulose were carefully added to the IL in 10 mg increments. After each addition step, a clear and visually homogeneous solution was awaited before proceeding to the next addition. The cellulose was incubated in the oil bath for 4 h at 110 °C. After cooling to room temperature, the cellulose was regenerated by the addition of distilled water followed by vigorous stirring for 30 min. The ensuing cellulose precipitates were collected by vacuum filtration, followed by thorough washing and drying using distilled water followed by acetone. The pretreated cellulose was dried in air for 48 h before use in experiments. (2) Suspension pretreatment. In cases where IL solutions could not fully dissolve cellulose, 0.2 g of cellulose (or 0.1 g of switchgrass) was suspended in 2.0 mL of an IL (or its aqueous solution) and incubated in an oil bath at 110 °C for 4 h. After the solution was cooled to room temperature, distilled water was added into the solution with vigorous stirring for 30 min. The treated cellulose was collected, washed, and dried exactly as written for the regeneration pretreatment approach.2.3 Enzymatic hydrolysis of cellulose and switchgrass
A suspension of 20 mg (untreated or pretreated) of Avicel PH-101 cellulose or switchgrass (2% wt/v loading) in 1.0 mL of citrate buffer solution (50 mM, pH 4.8) was incubated in a water bath at 50 °C under gentle stirring. The reaction was initiated by adding 3.0 mg (or 20 U) of Trichoderma reesei cellulase and 1.0 mg (or 3811 U) of β-glucosidase. An aliquot of 50 μL of the reaction mixture was periodically withdrawn and diluted with citrate buffer. The glucose concentration was determined by the glucose HK assay method at 340 nm using a Shimadzu UV-Mini-1240 spectrophotometer.25 The xylose concentration was determined by the D-xylose assay kit spectrophotometrically at 340 nm.26 All experiments were run in duplicate.2.4 XRD And SEM characterization
XRD patterns for untreated and IL-pretreated Avicel celluloses were analyzed at 25 °C by a D8-Focus X-ray diffraction system (Bruker AXS GmbH) using CuKα radiation (λ = 1.542 Å) generated at 40 kV and 40 mA. Samples were scanned over the angular range 2θ = 6–45°, with a step size of 0.02° and a step time of 0.5 s. The morphologies of the cellulosic materials, with or without IL pretreatment, were examined by scanning electron microscopy (S-4800, Hitachi, Ltd, Japan) at a 3 kV accelerating voltage.2.5 Thermogravimetric analysis
Thermogravimetric analysis (TGA) was performed with a TA Instruments TGA Q50 or TGA Q500 under a nitrogen atmosphere (40 mL min−1) using Pt pans and scanning from 20 to 600 °C at a heating rate of 10 °C min−1. The decomposition temperature Tdcp denotes the thermal onset of decomposition, signifying the occurrence of a 10% total mass loss during TGA scanning. Tder is another measure of decomposition temperature, which is defined as the maximum in the first-derivative envelope of the TGA scan.2.6 Cellulase adsorption isotherm
A known amount of cellulase was dissolved in 1.0 mL citrate buffer (pH 4.8, 50 mM) at 4 °C. The cellulose powder (20 mg) was added into the enzyme solution. The suspension solution was stirred continuously at 4 °C. After 30 min, the suspension solution was centrifuged to settle the insoluble cellulose. Thirty microliters (30 μL) of the supernatant were added into a curvet containing 1.5 mL of Bradford reagent. After mixing the solution at room temperature for 10 min, the protein concentration was determined by its absorbance at 595 nm (blank was 30 μL citrate buffer in 1.5 mL Bradford reagent). A protein calibration curve was generated from standard solutions of bovine serum albumin (BSA). The amount of adsorbed protein on cellulose was calculated from subtracting the remaining protein in solution after 30 min from the initial protein content.3 Results and discussion
3.1 Screening of ILs for cellulose pretreatment and the hydrolysis rate model
In this work, we chose Avicel PH-101 as our model cellulose because it represents a well-studied microcrystalline cellulose (MCC). We tested a wide variety of ionic solvent systems for cellulosic pretreatment, including 20 different ILs and three cholinium-based deep eutectic solvents (see Table S1 in ESI†) in Avicel cellulose pretreatment; the ionic solvents employed were either neat (anhydrous) or diluted in water to 1.0 or 2.0 M concentrations. Among those ILs examined, ones containing the chloride, acetate, or dimethylphosphate anion (viz., entries 1, 5, 13, and 16–20 in Table 1) appeared most adequate for dissolving appreciable amounts of cellulose (typically, 5–10 wt%). We further carried out enzymatic hydrolysis on native Avicel PH-101 or cellulose pretreated with the 23 different ionic solvent systems (neat and as 1.0 and 2.0 M solutions in water; 69 total solvent systems); typical hydrolysis kinetics profiles are illustrated in Fig. S1 (ESI†) using the example of [BMIM][OAc]. As shown in Fig. S1,† the hydrolysis of cellulose regenerated from neat [BMIM][OAc] was much faster that cellulose pretreated by aqueous [BMIM][OAc] solutions.| Ionic solvent | Vmax/Km (g L−1 h−1) and (R2)b | |||||
|---|---|---|---|---|---|---|
| Neat | 2.0 M | 1.0 M | ||||
| a Hydrolysis conditions: 1.0 mL citrate buffer (pH 4.8, 50 mM), 0.02 g untreated or pretreated Avicel PH-101, 3.0 mg Trichoderma reesei cellulase and 1.0 mg β-glucosidase under gentle agitation at 50 °C.b Calculated from hydrolysis data using the Michaelis–Menten equation (the number in parenthesis represents R2).c Italicized data indicate regeneration pretreatment and others are suspension pretreatments; bold Vmax/Km values indicate significantly high hydrolysis rates.d Cellulose was partially dissolved in neat IL. | ||||||
| None (untreated) | 0.10 (0.984) | |||||
| Different anions | ||||||
| 1 | [BMIM]Cl | 0.22c (0.972) | 0.12(0.990) | 0.15 (0.980) | ||
| 2 | [BMIM]Br | 0.12d (0.989) | 0.13 (0.991) | 0.11 (0.993) | ||
| 3 | [BMIM][BF4] | 0.11 (0.985) | 0.13 (0.996) | 0.12 (0.992) | ||
| 4 | [BMIM][CF3COO] | 0.11 (0.998) | 0.10 (0.991) | 0.10 (0.991) | ||
| 5 | [BMIM][OAc] | 0.46 (0.980) | 0.13 (0.976) | 0.14 (0.971) | ||
| 6 | [BMIM][OTf] | 0.065 (0.991) | 0.078 (0.994) | 0.083 (0.975) | ||
| 7 | [BMIM][MeSO3] | 0.093 (0.988) | 0.16 (0.985) | 0.14 (0.985) | ||
| 8 | [BMIM][HSO4] | 0.036 (0.978) (4.0 M) | 0.081 (0.968) | 0.090 (0.975) | ||
| 9 | [BMIM][SCN] | 0.13 (0.980) | 0.14 (0.996) | 0.14 (0.989) | ||
| 10 | [BMIM][dca] | 0.11 (0.989) | 0.10 (0.987) | 0.11 (0.995) | ||
| 11 | [BMIM][NO3] | 0.13 (0.988) | 0.11 (0.988) | 0.12 (0.984) | ||
| 12 | [BMIM][MeSO4] | 0.12 (0.995) | 0.11 (0.994) | 0.12 (0.993) | ||
| 13 | [BMIM][Me2PO4] | 0.42 (0.990) | 0.12 (0.987) | 0.12 (0.966) | ||
| 14 | [BMIM][PF6] | 0.088 (0.977) | 0.10 (0.976) | 0.13 (0.989) | ||
| 15 | [BMIM][Tf2N] | 0.13 (0.983) | 0.13 (0.960) | 0.13 (0.982) | ||
| Different cations | ||||||
| 16 | [EMIM][OAc] | 0.44 (0.927) | 0.14 (0.970) | 0.12 (0.992) | ||
| 5 | [BMIM][OAc] | 0.46 (0.980) | 0.13(0.976) | 0.14 (0.971) | ||
| 17 | [HMIM][OAc] | 0.21 (0.974) | 0.13 (0.984) | 0.13 (0.992) | ||
| 18 | [CH3(OCH2CH2)3–Et-Im][OAc] | 0.25 (0.995) | 0.10 (0.981) | 0.12 (0.990) | ||
| 19 | [CH3(OCH2CH2)2–Et3N][OAc] | 0.34 (0.955) | 0.13 (0.998) | 0.13 (0.991) | ||
| 20 | [CH3(OCH2CH2)3–Et-Pip][OAc] | 0.40 (0.960) | 0.10 (0.960) | 0.15 (0.985) | ||
| Deep eutectic solvents (DES) | ||||||
| 21 | Choline chloride/urea (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) 2) |
0.085 (0.998) | 0.10 (0.988) | 0.10 (0.967) | ||
| 22 | Choline chloride/glycerol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) 2) |
0.13 (0.950) | 0.11 (0.986) | 0.14 (0.997) | ||
| 23 | Choline acetate/glycerol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5) 1.5) |
0.10 (0.982) | 0.11 (0.987) | 0.13 (0.970) | ||
To quantitatively compare the hydrolysis rates, a simple Michaelis–Menten model was used to evaluate the hydrolysis reaction rate. Although cellulase is a cocktail consisting of several major enzymes (such as endoglucanase, exoglucanase and β-glucosidases), many studies have suggested that the Michaelis–Menten equation (competitive or noncompetitive inhibition)27–29 or its modifications (such as kinetics with competitive inhibition and Langmuir adsorption)30,31 could describe the kinetics of enzymatic hydrolysis of cellulose. Therefore, this study adopts the Michaelis–Menten equation (eqn (1)) to curve-fit our hydrolysis data.
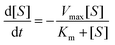 | (1) |
Here, [S] is the concentration of substrate (i.e. cellulose) which can be estimated from the initial cellulose concentration (20 g L−1) and time-dependent glucose concentration (determined by the glucose HK assay periodically); Vmax represents the maximum hydrolysis rate; Km is the substrate concentration at which the reaction rate is half of Vmax; Vmax/Km represents the reaction rate constant at a low substrate concentration. The Runge–Kutta algorithm (see ESI†) was used in the curve-fitting analysis with the Microsoft Excel GRG nonlinear method to obtain the Vmax and Km values for each hydrolysis reaction within 5 h. The Vmax/Km values are compiled in Table 1. The R2 values are in the range of 0.927–0.998 and most of them fall between 0.97 and 0.99, which suggests the simple the Michaelis–Menten equation correlates the hydrolysis data quite well. The results for the ionic solvents shown in Table 1 are divided into three distinct categories: (i) ILs based on the [BMIM]+ cation with different anions, (ii) ILs based on the acetate (OAc−) anion paired with different cations, and (iii) deep eutectic solvents (DESs). As a benchmark, untreated Avicel showed a Vmax/Km value of 0.10 g L−1 h−1, and this forms the basis for comparing how various IL-pretreatments influence cellulose hydrolysis.
3.2 Impact of IL properties on cellulose pretreatment
At first, we noticed that Avicel samples regenerated from cellulose-dissolving ILs (i.e., 1, 5, 13, 16–20) displayed much higher hydrolysis rates (Vmax/Km ranging from 0.21 to 0.46 g L−1 h−1) than untreated cellulose (0.10 g L−1 h−1). As has been explained in earlier studies, cellulose regenerated from an IL is more accessible to cellulase action due to the reduction in crystallinity, a transition from cellulose I to cellulose II structure, as well as other factors.10,32–34 In this study, we are interested in learning how different anions and cations within neat ILs, as well as aqueous IL solutions, influence cellulose accessibility and hydrolysis. In order to facilitate a general discussion of trends in the anion's influence on cellulose hydrolysis rates, we visualize our results for a series of [BMIM]+ ILs in Fig. 1. Broadly, for each ionic solvent condition (i.e., neat, 1.0 M (aq), and 2.0 M (aq)) we can categorize the ILs into three groups: (i) those achieving hydrolysis rates for treated Avicel much faster than that observed for untreated Avicel, (ii) those reaching only slightly faster rates, and (iii) those showing a similar hydrolysis rate as the untreated cellulose. | ||
| Fig. 1 Summary of anion effect on the hydrolysis rates of Avicel PH-101 pretreated by [BMIM]+ type ILs. | ||
When neat ILs were used in the pretreatment of cellulose, those with the “cellulose-dissolving” anions OAc−, Me2PO4− and Cl− gave the fastest reaction rates (Vmax/Km = 0.21 – 0.46 g L−1 h−1). ILs containing NO3−, SCN−, Tf2N−, MeSO4− and Br− anions yielded only moderately improved rates (Vmax/Km = 0.12 – 0.13 g L−1 h−1) while Avicel treated with ILs containing the remaining anions (dca−, CF3COO−, BF4−, MeSO3−, PF6− and OTf−) showed about the same hydrolysis rates as the untreated cellulose. The high hydrogen-bond basicity of certain anions (as designated by a high Kamlet–Taft β parameter) has been credited with breaking up key inter- and intra-molecular hydrogen-bonds within cellulose, leading to biomass dissolution in favorable ILs.24,35,36 As illustrated in Fig. 2, a correlation between the observed Avicel hydrolysis rates and the Kamlet–Taft β value exists for cellulose treated with neat ILs. In general, a higher hydrogen-bond basicity coincides with a higher hydrolysis rate, although exceptions such as OTf− and Tf2N− are apparent. In addition, we established a weak correction between the hydrolysis rate and the polarity of neat ILs (see Fig. 3): a lower IL polarity correlates to a faster hydrolysis. A likely explanation is that favorable cellulose solvation and dissolution require balanced van der Waals forces and hydrophobic interactions,37 which is only partially reflected in the empirical polarity determined by Reichardt's dye. Conversely, as shown in Fig. S2 and S3,† the Hofmeister ranking of anions (as characterized by viscosity B-coefficients) and the hydrophobicity of ILs (as quantified by log P values) have no direct correlation with the hydrolysis rate of cellulose pretreated by neat ILs.
 | ||
Fig. 2 Effect of hydrogen-bond basicities of [BMIM]+-based ILs on the hydrolysis rate of Avicel PH-101. [Data references for IL Kamlet–Taft β-values: OAc−,24 Cl−,42 MeSO4−, and SCN−,43 OTf−, BF4−, Tf2N−, and PF6−![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 44]. 44]. | ||
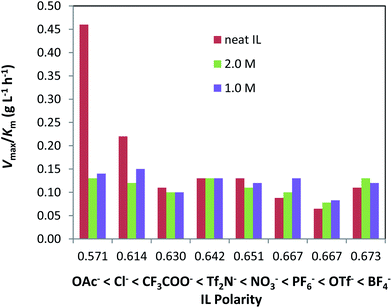 | ||
Fig. 3 Effect of polarity of [BMIM]+-based ILs on the hydrolysis rate of Avicel PH-101. [Data references for ENT polarity values: OAc−, CF3COO−, and NO3−,45 Cl−,46 and Tf2N−, PF6−, OTf−, and BF4−![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 47]. 47]. | ||
Water is known as an “anti-solvent” in cellulose dissolution because the addition of water rapidly precipitates out cellulose initially dissolved in an IL. Aqueous solutions of ILs (such as our 1.0 and 2.0 M solutions studies here) cannot dissolve cellulose. Consequently, the pretreatment of cellulose by aqueous ILs is referred to as “suspension pretreatment”. As shown in Table 1, for a 2.0 M concentration, ILs comprising the anions MeSO3−, SCN−, Tf2N−, BF4−, Br− and OAc− gave the fastest hydrolysis with Vmax/Km ranging from 0.13 to 0.16 g L−1 h−1. ILs based on Cl−, Me2PO4−, MeSO4− and NO3− led to moderate rate increases (Vmax/Km = 0.11–0.12 g L−1 h−1) whilst the remaining ILs (CF3COO−, dca−, PF6−, HSO4− and OTf−) actually showed about the same (or slightly lower) hydrolysis rate as untreated Avicel.
When these same ILs are employed at a lower concentration of 1.0 M, their relative impact on cellulose hydrolysis differs from the case of 2.0 M. At 1.0 M, ILs with Cl−, MeSO3−, OAc−, SCN−, PF6− and Tf2N− anions resulted in the fastest reactions (Vmax/Km = 0.13–0.15 g L−1 h−1), those containing MeSO4−, NO3−, BF4−, Me2PO4−, dca− and Br− gave modestly improved rates (Vmax/Km = 0.11–0.12 g L−1 h−1), and others (CF3COO−, HSO4−,and OTf−) caused no appreciable changes to the reaction rate. In contrast to the use of neat ILs for pretreatment, aqueous ILs show strikingly different effects on the Avicel hydrolysis rate and no clear or direct correction between the hydrolysis rate and simple physicochemical properties could be established, including hydrogen-bond basicity (Fig. 2), polarity (Fig. 3), Hofmeister classification (Fig. S2†), or hydrophobicity (Fig. S3†).
As highlighted in Table 1, pretreatment by several aqueous IL solutions proved effective for improving the hydrolysis rate of cellulose. These solutions included [BMIM]Cl (1.0 M: Vmax/Km = 0.15 g L−1 h−1), [BMIM][OAc] (1.0 M: Vmax/Km = 0.14 g L−1 h−1), [BMIM][MeSO3] (1.0 M: Vmax/Km = 0.14 g L−1 h−1; 2.0 M: Vmax/Km = 0.16 g L−1 h−1), and [BMIM][SCN] (1.0 and 2.0 M: Vmax/Km = 0.14 g L−1 h−1). An earlier study by Brandt et al.22 suggested that 80% (v/v) aqueous solutions of [BMIM][HSO4], [BMIM][MeSO3], and [BMIM][MeSO4] were effective for the delignification and improved digestibility of cellulose. These authors also indicated that no correlation exists between the pretreatment effectiveness and the hydrogen-bond basicity of the ILs. In our hands, neat [BMIM][HSO4] caused considerable cellulose degradation at 110 °C, resulting in little recovered cellulose. For this reason, the highest level of [BMIM][HSO4] used was 4.0 M, as noted in Table 1. In either case, [BMIM][HSO4] proved ineffective in generating a rapid cellulose hydrolysis.
It emerges from the entries in Table 1 and Fig. 1 that pretreatment by several IL systems actually causes a slight reduction (Vmax/Km below 0.10 g L−1 h−1) in cellulose hydrolysis. One probable reason is that residual ILs in IL-treated cellulose might lead to cellulase inactivation. As shown in Table S1,† the calculated cellulose recovery may actually exceed 100%, especially among regenerated celluloses. This is an indication that IL residues likely become embedded within cellulose chains and resist efforts to wash it away. In an earlier study, we were able to both detect residual ILs after the complete hydrolysis of cellulose and to confirm that the presence of ILs inactivated the cellulase.33
The cationic structure of the IL may also moderate the pretreatment efficiency. As suggested by the entries of Table 1, the hydrolysis rate of cellulose pretreated by [HMIM][OAc] (17) is slower than those pretreated by [EMIM][OAc] (16) and [BMIM][OAc] (5). There are two likely reasons for this: (1) firstly, a longer alkyl chain leads to a larger molar volume, effectively lowering the anion concentration, which in turn minimizes the disruptive capability of the anion.9 (2) In addition, the residual IL lingering in the recovered cellulose (see Table S1†) may prompt dissimilar degrees of cellulase deactivation. Notably, the three ILs prepared from cations bearing ether functionality (18, 19, 20) are capable of dissolving cellulose and enabling its fast enzymatic hydrolysis (Vmax/Km = 0.25–0.40 g L−1 h−1) (Table 1). These ether-containing ILs developed previously in our group9,10,38 have the advantages of relatively low viscosity and low cost, potentially high biodegradability, and minimal cellulase inactivation, the latter due in part to the low anion concentration.
Cousins to the conventional IL, deep eutectic solvents (DESs, frequently choline based) are extensions of and alternatives to ILs and deliver several attractive properties such as low cost, low toxicity, high biodegradability and high enzyme compatibility.39 For this reason, we examined three DESs (21, 22, 23) and their aqueous solutions in the pretreatment of Avicel cellulose (Table 1). Encouragingly, we found that some DESs are as effective as aqueous ILs in pretreating cellulose and improving its subsequent hydrolysis. Further, in aqueous solutions of choline chloride (or acetate) mixed with glycerol, we measured Vmax/Km values of 0.14 and 0.13 g L−1 h−1 in 1.0 M solutions of 22 and 23, respectively. Remarkably, we also witnessed that a 1.0 M solution outperforms a 2.0 M solution, the latter frequently giving no advantage over untreated Avicel. Overall, these results suggest a largely untapped potential for aqueous DESs in the pretreatment of cellulose.
3.3 Cellulose structural characterization by XRD, SEM, TGA and adsorption isotherm
To better elucidate how ionic solvents perturb the cellulosic structure, we performed X-ray diffraction (XRD), scanning electron microscopy (SEM), and thermogravimetric analysis (TGA) analysis on untreated Avicel alongside several representative treated samples. As shown in Fig. 4a, XRD studies indicate that cellulose regenerated from [BMIM][OAc] has a much lower crystallinity (35%) than untreated Avicel (79%). In contrast, cellulose samples pretreated by aqueous [BMIM][OAc] (1.0 and 2.0 M) exhibit no such reduction in crystallinity. The changes in cellulose crystallinity correlate nicely with the enzymatic hydrolysis rate of cellulose (Table 1). Earlier studies10,40 showed that cellulosic regeneration from an IL is often accompanied by a change in structure from cellulose I to cellulose II. Representative SEM images further corroborate that the morphology of untreated cellulose (Fig. 5a) is more heterogeneous consisting of large particles, while the regenerated cellulose (Fig. 5b) appears to be more homogeneous and uniform; celluloses treated by aqueous [BMIM][OAc] (1.0 and 2.0 M) (Fig. 5c and d) seem to be in-between the heterogeneous and homogeneous morphologies. In the case of [BMIM][MeSO3], however, Avicel cellulose pretreatment gave no reduction in crystallinity (Fig. 4b) for either neat IL or aqueous ILs. From these results, it should be clear that the cellulose structural changes and accessibility to cellulase cannot be solely predicted from the XRD crystallinity index.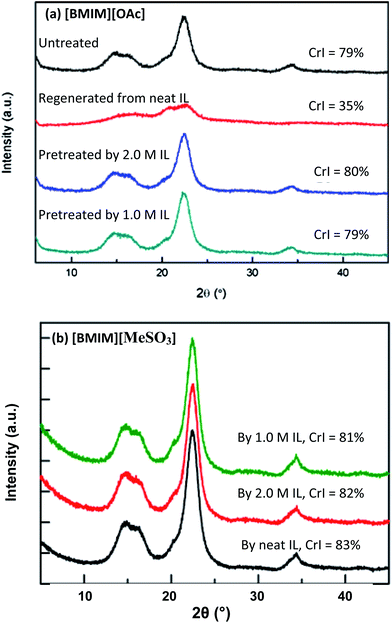 | ||
| Fig. 4 XRD patterns for untreated Avicel PH-101 cellulose and Avicel pretreated by (a) [BMIM][OAc] or (b) [BMIM][MeSO3]. | ||
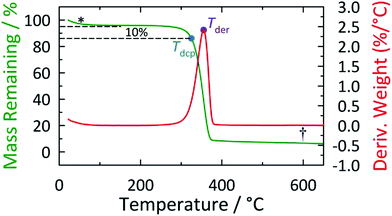
The alteration in cellulose structure caused by pretreatment can also be reflected in the thermal stability of the resultant cellulose. Toward this, we conducted TGA experiments on cellulose samples prior to and following various pretreatment strategies. Two representative stability parameters are given in Table 2: (i) the decomposition temperature (Tdcp) denotes the thermal onset of decomposition at a 10% total mass loss; and (ii) Tder is the maximum displayed in the first-derivative of the TGA scan. We note that although Tdcp is most seen in the recent literature, we provide both indices for completeness. Untreated cellulose displays a fairly high Tdcp of 323 °C. Typically, cellulose pretreatment results in structural changes which reduce Tdcp.10 Thus, to an extent, the Tdcp value reflects the severity of structural changes occurring as a result of pretreatment. As indicated in Table 2, regenerated cellulose from [BMIM][OAc] has a much lower Tdcp (222 °C) than cellulose pretreated by aqueous [BMIM][OAc] solution (∼300 °C). This accords with our earlier XRD and SEM results, as well as the observed hydrolysis rates. Namely, the process of dissolving cellulose in neat [BMIM][OAc] significantly modifies the cellulose crystallinity and makes the cellulose more susceptible to hydrolysis whereas aqueous [BMIM][OAc] has minimal impact on the cellulose structure. However, this analysis appears less applicable in the case of [BMIM][MeSO3] pretreatment. Specifically, cellulose pretreated by neat [BMIM][MeSO3] shows a significant reduction in thermal stability (Tdcp = 281 °C), however, Vmax/Km is only 0.093 g L−1 h−1 in this case. Contrariwise, cellulose samples treated by aqueous [BMIM][MeSO3] show essentially an identical Tdcp as untreated Avicel (Table 2 and Fig. S5†). However, cellulose hydrolysis kinetics analysis clearly reveals a favorable boost upon pretreatment with aqueous [BMIM][MeSO3] (1.0 M: Vmax/Km = 0.14 g L−1 h−1; 2.0 M: Vmax/Km = 0.16 g L−1 h−1). From this, we can conclude that XRD and TGA studies may not be able to reveal some subtle but important structural changes that occur during cellulose pretreatment with ILs and their aqueous solutions.
| Sample pretreatment | Early mass lossb | Tder/°Cc | Tdcp/°Cd | Transition shapee | Residual charf |
|---|---|---|---|---|---|
| a TGA scans were measured on a TA Instruments TGA Q50 under a nitrogen atmosphere using Pt pans with a heating rate of 10 °C min−1. See footer graphic for a diagram illustrating the measurement of the various transitions and mass loss steps tabulated; * and † indicate the early mass loss step and the position for determination of residual char, respectively.b The early mass loss at temperatures below 100 °C is attributed primarily to buoyancy effects and, for this reason, is disregarded in the estimation of Tdcp.c Tder is determined from the maximum in the first-derivative envelope of the TGA scan.d Tdcp is the decomposition temperature measured at the onset of decomposition (i.e., 10% mass loss following the initial plateau beyond the early mass loss).e The main mass loss behavior is characterized on the basis of whether it occurs as a single, discrete step (S) or exhibits multi-step (M) thermal decomposition. (B) Indicates a derivative thermogram which essentially occurs as a single step but with a slightly bimodal appearance, such as a pronounced shoulder (see Fig. S4–S6†).f The amount of carbonaceous char is determined from the mass remaining at 600 °C; uncertainties are estimated to be on the order of ±1–2%.g In contrast to the other samples, the residual char observed for this particular sample showed a large variability. | |||||
| Avicel, untreated | 5.6% | 345 | 323 | S | 1.1% |
| Avicel, neat [BMIM][OAc] | 4.8% | 280 | 222 | M | 24.8% |
| Avicel, 2.0 M [BMIM][OAc] | 4.7% | 370 | 300 | S | 8.7% |
| Avicel, 1.0 M [BMIM][OAc] | 4.3% | 370 | 301 | S | 3.0% |
| Avicel, neat [BMIM][MeSO3] | 4.7% | 323 | 281 | S | 6.9% |
| Avicel, 2.0 M [BMIM][MeSO3] | 4.1% | 355 | 325 | S | 6.6% |
| Avicel, 1.0 M [BMIM][MeSO3] | 4.9% | 351 | 323 | S | 4.6% |
| Switchgrass, untreated | 5.2% | 332 | 250 | B | 22.0% |
| Switchgrass, neat [BMIM][OAc] | 10.9% | 294 | 232 | B | 15.4% |
| Switchgrass, 2.0 M [BMIM][OAc] | 8.0% | 351 | 250 | B | 5.3% |
| Switchgrass, 1.0 M [BMIM][OAc] | 3.6% | 346 | 248 | B | 17.3%g |
 |
|||||
To probe such subtle structural changes, we carried out the cellulase adsorption isotherm of Avicel celluloses pretreated by various ionic solutions (Fig. 6 and S7†). The adsorption experiments were carried out at 4 °C to minimize the enzymatic hydrolysis of cellulose. The adsorption isotherm measures the cellulase-binding capability of cellulose, which further implies the porousness and accessibility of cellulose. As shown in Fig. 6, celluloses pretreated by aqueous [BMIM][MeSO3] (1.0 and 2.0 M) exhibit a higher enzyme-adsorption capacity than that treated by neat [BMIM][MeSO3], suggesting the aqueous IL-pretreatment leads to a higher accessible surface area and more binding sites for cellulase. This explains the higher hydrolysis rates of celluloses pretreated by aqueous [BMIM][MeSO3] than that by neat IL (Table 1). Fig. S7† indicates no significant adsorption differences among cellulose samples pretreated by neat or aqueous choline chloride/glycerol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2). Although the exact mechanism of how aqueous ILs interact with cellulose chains is not clear, it is helpful to offer some insightful discussions. When neat or aqueous ILs are not capable of dissolving cellulose, these solvent molecules may not considerably disrupt the hydrogen-bonding network to make noticeable structural changes as detected by XRD and TGA. However, in aqueous ILs, hydrated ions may have better penetration/diffusion power than (non-cellulose dissolving) neat IL molecules to weakly disrupt the hydrogen-bonds, enable subtle morphology changes, and increase the cellulose accessibility and digestibility.
2). Although the exact mechanism of how aqueous ILs interact with cellulose chains is not clear, it is helpful to offer some insightful discussions. When neat or aqueous ILs are not capable of dissolving cellulose, these solvent molecules may not considerably disrupt the hydrogen-bonding network to make noticeable structural changes as detected by XRD and TGA. However, in aqueous ILs, hydrated ions may have better penetration/diffusion power than (non-cellulose dissolving) neat IL molecules to weakly disrupt the hydrogen-bonds, enable subtle morphology changes, and increase the cellulose accessibility and digestibility.
 | ||
| Fig. 6 T. reesei cellulase adsorption isotherm (4 °C) of Avicel PH-101 pretreated by neat or aqueous [BMIM][MeSO3]. | ||
3.4 IL pretreatment of switchgrass
Switchgrass (Panicum virgatum L.) is known to be a viable source of lignocellulose for bioethanol production.41 Given its relevance to bioenergy, we initiated studies pretreating switchgrass with [BMIM][OAc], [BMIM][MeSO3], and their aqueous solutions (Fig. 7 and 8). As shown in Fig. 7 and 8, pretreatment by both neat [BMIM][OAc] (which partially dissolves the grass) and [BMIM][MeSO3] significantly sped up the enzymatic hydrolysis of cellulose. In addition, pretreatment with neat [BMIM][OAc] improved the hydrolysis of hemicellulose. Aqueous [BMIM][OAc] solutions proved to be much less effective for pretreating switchgrass and gave low hydrolysis rates for both cellulose and hemicellulose. XRD patterns (Fig. 9a) confirm a substantially reduced crystallinity (23%) for switchgrass regenerated from neat [BMIM][OAc] (cf. 41% for untreated grass), and a lower, but still substantial, reduction in crystallinity following aqueous [BMIM][OAc] treatment (1.0 M: 27%; 2.0 M: 30%). The associated SEM images shown in Fig. 10 indicate that switchgrass samples pretreated by aqueous [BMIM][OAc] retain a heterogeneous morphology similar to the untreated grass, while the grass regenerated from neat [BMIM][OAc] appears to be more homogeneous. | ||
| Fig. 9 XRD patterns for untreated switchgrass and switchgrass pretreated by (a) [BMIM][OAc] and (b) [BMIM][MeSO3]. | ||
Alternatively, aqueous [BMIM][MeSO3] solutions proved surprisingly effective as pretreatment agents to produce high hydrolysis rates for both cellulose and hemicellulose. These observations are in agree with our above results demonstrating that aqueous [BMIM][MeSO3] is a promising treatment medium for processing Avicel cellulose. However, as revealed in Fig. 9b, there is no perceptible reduction in crystallinity observed for switchgrass samples pretreated by either neat [BMIM][MeSO3] or aqueous [BMIM][MeSO3]. This observation supports the notion that, in spite of no evident change in crystallinity, certain pretreatments can lead to subtle changes that markedly impact the cellulose accessibility and digestibility.
4 Conclusions
In this research, we have systematically studied the hydrolysis rates of cellulose and switchgrass pretreated by neat ILs, DESs, and their aqueous solutions. The hydrolysis rate of cellulose treated by neat ILs can be correlated with the H-bond basicity of the constituent anions and the empirical IL polarity, however, the hydrolysis rates of cellulose pretreated by aqueous ILs cannot be simply related to any single property of ILs (e.g., H-bond basicity, polarity, Hofmeister ranking, and hydrophobicity). We further examined the structural changes of cellulosic biomass upon pretreatment by IL solutions using XRD, SEM, TGA and adsorption isotherm characterizations. In the case of [BMIM][OAc], the hydrolysis rates for Avicel and switchgrass correspond to observable structural changes: particularly, a reduction in crystallinity, a homogeneous morphology, and a reduced thermal stability. However, in the case of [BMIM][MeSO3], the hydrolysis rates of (ligno)cellulose can be related to neither the crystallinity nor the thermal stability of the cellulose; but a higher cellulase-adsorption trend was observed for celluloses pretreated by aqueous IL solutions than that by neat IL. Clearly, multifaceted or elusive structural changes must be implicated in this case. From this work, aqueous solutions of several ILs and DESs emerge as effective pretreatment agents for cellulose, including [BMIM][MeSO3] (7), [CH3(OCH2CH2)3–Et-Pip][OAc] (20), [BMIM][OAc] (5), [EMIM][OAc] (16), [BMIM]Cl (1), [BMIM][SCN] (9), as well as choline chloride/glycerol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) (22). In short, aqueous ILs and aqueous DESs represent promising and inexpensive alternative solvent systems for pretreating lignocellulose to attain rapid saccharification rates of both cellulose and hemicellulose. In this regard, we anticipate that our present results will embolden much-needed consideration and activity in this underexplored area.
2) (22). In short, aqueous ILs and aqueous DESs represent promising and inexpensive alternative solvent systems for pretreating lignocellulose to attain rapid saccharification rates of both cellulose and hemicellulose. In this regard, we anticipate that our present results will embolden much-needed consideration and activity in this underexplored area.
Acknowledgements
The authors declare no potential sources of conflict of interest. HZ acknowledges supports by the Henry Dreyfus Teacher-Scholar Award (2012), NIH MBRS-RISE grant (1R25GM096956), and NIH NIBIB contract award (HHSN268201200011C).References
- M. Mora-Pale, L. Meli, T. V. Doherty, R. J. Linhardt and J. S. Dordick, Biotechnol. Bioeng., 2011, 108, 1229–1245 CrossRef CAS PubMed.
- A. Stark, Energy Environ. Sci., 2011, 4, 19–32 CAS.
- T. Vancov, A.-S. Alston, T. Brown and S. McIntosh, Renewable Energy, 2012, 45, 1–6 CrossRef CAS PubMed.
- H. Tadesse and R. Luque, Energy Environ. Sci., 2011, 4, 3913–3929 CAS.
- H. Wang, G. Gurau and R. D. Rogers, Chem. Soc. Rev., 2012, 41, 1519–1537 RSC.
- S. H. Lee, T. V. Doherty, R. J. Linhardt and J. S. Dordick, Biotechnol. Bioeng., 2009, 102, 1368–1376 CrossRef CAS PubMed.
- C. Mukesh, D. Mondal, M. Sharma and K. Prasad, Chem. Commun., 2013, 49, 6849–6851 RSC.
- D. Mondal, M. Sharma, C. Mukesh, V. Gupta and K. Prasad, Chem. Commun., 2013, 49, 9606–9608 RSC.
- H. Zhao, G. A. Baker, Z. Song, O. Olubajo, T. Crittle and D. Peters, Green Chem., 2008, 10, 696–705 RSC.
- S. Tang, G. A. Baker, S. Ravula, J. E. Jones and H. Zhao, Green Chem., 2012, 14, 2922–2932 RSC.
- N. Muhammad, Z. Man, M. A. Bustam, M. I. Abdul Mutalib, C. D. Wilfred and S. Rafiq, Appl. Biochem. Biotechnol., 2011, 165, 998–1009 CrossRef CAS PubMed.
- K. Ohira, Y. Abe, M. Kawatsura, K. Suzuki, M. Mizuno, Y. Amano and T. Itoh, ChemSusChem, 2012, 5, 388–391 CrossRef CAS PubMed.
- A. W. T. King, J. Asikkala, I. Mutikainen, P. Järvi and I. Kilpeläinen, Angew. Chem., Int. Ed., 2011, 50, 6301–6305 CrossRef CAS PubMed.
- Q. Zhang, N. S. Oztekin, J. Barrault, K. De Oliveira Vigier and F. Jérôme, ChemSusChem, 2013, 6, 593–596 CrossRef CAS PubMed.
- Q. Zhang, M. Benoit, K. De Oliveira Vigier, J. Barrault and F. Jérôme, Chem.–Eur. J., 2012, 18, 1043–1046 CrossRef CAS PubMed.
- R. Rinaldi, Chem. Commun., 2011, 47, 511–513 RSC.
- M. Francisco, A. van den Bruinhorst and M. C. Kroon, Green Chem., 2012, 14, 2153–2157 RSC.
- M. Gericke, T. Liebert, O. A. El Seoud and T. Heinze, Macromol. Mater. Eng., 2011, 296, 483–493 CrossRef CAS.
- X. Tian, Z. Fang, D. Jiang and X. Sun, Biotechnol. Biofuels, 2011, 4, 53 CrossRef CAS PubMed.
- D. Fu and G. Mazza, Bioresour. Technol., 2011, 102, 7008–7011 CrossRef CAS PubMed.
- D. Fu and G. Mazza, Bioresour. Technol., 2011, 102, 8003–8010 CrossRef CAS PubMed.
- A. Brandt, M. J. Ray, T. Q. To, D. J. Leak, R. J. Murphy and T. Welton, Green Chem., 2011, 13, 2489–2499 RSC.
- Z. Zhang, I. M. O'Hara and W. O. S. Doherty, Bioresour. Technol., 2012, 120, 149–156 CrossRef CAS PubMed.
- H. Ohno and Y. Fukaya, Chem. Lett., 2009, 38, 2–7 CrossRef CAS.
- R. J. L. Bondar and D. C. Mead, Clin. Chem., 1974, 20, 586–590 CAS.
- H. Zhao, G. A. Baker and J. V. Cowins, Biotechnol. Prog., 2010, 26, 127–133 CAS.
- C. P. Dwivedi and T. K. Ghose, J. Ferment. Technol., 1979, 57, 15–24 CAS.
- G. Caminal, J. López-Santín and C. Solà, Biotechnol. Bioeng., 1985, 27, 1282–1290 CrossRef CAS PubMed.
- T. K. Ghose and K. Das, in Advances in Biochemical Engineering 1, ed. T. K. Ghose and A. Fiechter, Springer-Verlag, New York, 1971, pp. 55–76 Search PubMed.
- A. A. Huang, Biotechnol. Bioeng., 1975, 17, 1421–1433 CrossRef CAS PubMed.
- L. T. Fan and Y. H. Lee, Biotechnol. Bioeng., 1983, 25, 2707–2733 CrossRef CAS PubMed.
- A. P. Dadi, S. Varanasi and C. A. Schall, Biotechnol. Bioeng., 2006, 95, 904–910 CrossRef CAS PubMed.
- H. Zhao, C. L. Jones, G. A. Baker, S. Xia, Z. Song, O. Olubajo and V. N. Person, J. Biotechnol., 2009, 139, 47–54 CrossRef CAS PubMed.
- S. Singh, B. A. Simmons and K. P. Vogel, Biotechnol. Bioeng., 2009, 104, 68–75 CrossRef CAS PubMed.
- Y. Fukaya, K. Hayashi, M. Wada and H. Ohno, Green Chem., 2008, 10, 44–46 RSC.
- T. V. Doherty, M. Mora-Pale, S. E. Foley, R. J. Linhardt and J. S. Dordick, Green Chem., 2010, 12, 1967–1975 RSC.
- N. P. Novoselov, E. S. Sashina, V. E. Petrenko and M. Zaborsky, Fibre Chem., 2007, 39, 153–158 CrossRef CAS.
- S. Tang, G. A. Baker and H. Zhao, Chem. Soc. Rev., 2012, 41, 4030–4066 RSC.
- H. Zhao and G. A. Baker, J. Chem. Technol. Biotechnol., 2013, 88, 3–12 CrossRef CAS.
- I. P. Samayam, B. L. Hanson, P. Langan and C. A. Schall, Biomacromolecules, 2011, 12, 3091–3098 CrossRef CAS PubMed.
- D. R. Keshwania and J. J. Cheng, Bioresour. Technol., 2009, 100, 1515–1523 CrossRef PubMed.
- Y. Fukaya, A. Sugimoto and H. Ohno, Biomacromolecules, 2006, 7, 3295–3297 CrossRef CAS PubMed.
- A. Oehlke, K. Hofmann and S. Spange, New J. Chem., 2006, 30, 533–536 RSC.
- L. Crowhurst, P. R. Mawdsley, J. M. Perez-Arlandis, P. A. Salter and T. Welton, Phys. Chem. Chem. Phys., 2003, 5, 2790–2794 RSC.
- J. L. Kaar, A. M. Jesionowski, J. A. Berberich, R. Moulton and A. J. Russell, J. Am. Chem. Soc., 2003, 125, 4125–4131 CrossRef CAS PubMed.
- J. G. Huddleston, G. A. Broker, H. D. Willauer and R. D. Rogers, in Ionic Liquids: Industrial Applications to Green Chemistry, ed. R. D. Rogers and K. R. Seddon, American Chemical Society, Washington, D. C., 2002, pp. 270–288 Search PubMed.
- M. J. Muldoon, G. M. Gordon and I. R. Dunkin, J. Chem. Soc., Perkin Trans. 2, 2001, 433–435 RSC.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c3ra46149a |
| ‡ In this case, 1 U corresponds to the liberation of 1.0 μg of glucose per minute at 35 °C. |
| This journal is © The Royal Society of Chemistry 2014 |




