Folate and biotin based bifunctional quantum dots as fluorescent cell labels†
Abstract
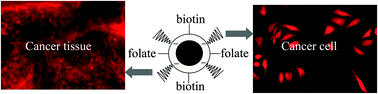
Although nanoprobes functionalized with one type of affinity molecule are commonly used as bioimaging probes, biolabeling studies of nanoprobes functionalized with more than one type of affinity molecule remains an unexplored area of research. Here we show that the labeling specificity of a nanoprobe can be enhanced if they are functionalized with more than one type of affinity molecule. We have synthesized functional quantum dots (QDs) of 20–30 nm hydrodynamic diameter having both folate and biotin on their surface. This dual functional nanoprobe has an enhanced labeling specificity to cancer cells/tissue as compared to folate or biotin based monofunctional QDs.

 Please wait while we load your content...
Please wait while we load your content...