Modulated fluorescence properties in fluorophore-containing gold nanorods@mSiO2†
Abstract
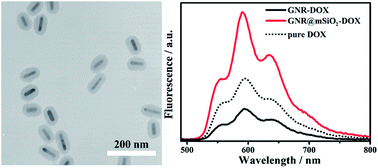
The influencing factors and mechanism of fluorescence enhancement or quenching in mesoporous nanocarriers consisting of gold nanorod cores and mesoporous silica shells (GNR@mSiO2) with controlled thicknesses were investigated. Mesoporous silica can act as a tool to change the distance between the fluorophores and GNRs as well as a vehicle to load the fluorescence molecules in this system. We found that the distance between the GNRs’ surface and the fluorophores, the distribution of fluorophores in mesoporous silica and the kind of fluorophore used were the three main reasons for the fluorescence change. The shell-thickness-dependent fluorescence enhancement of doxorubicin (DOX)-containing GNR@mSiO2 was observed. The maximum metal-enhanced fluorescence (MEF) was obtained when GNR@mSiO2–DOX had the thinnest mesoporous silica shell. Moreover, changing the concentration ratio between GNR@mSiO2 and DOX resulted in different fluorescence enhancement factors. Upon combination of GNR@mSiO2 with other fluorophores, such as hematoporphyrin dihydrochloride (HP) and rhodamine 6G (R6G), the fluorescence enhancement phenomenon was also observed. On the other hand, we found that the fluorescence enhancement factor was reduced when the emission wavelength of the fluorophores was generally close to the surface plasmon resonance wavelength of the gold nanorods. This mechanism was confirmed by fluorescence quenching on fluorescein isothiocyanate (FITC)-containing GNR@mSiO2.

 Please wait while we load your content...
Please wait while we load your content...