Facile synthesis of carbon quantum dots and thin graphene sheets for non-enzymatic sensing of hydrogen peroxide†
Mriganka Sadhukhan,
Tanmay Bhowmik,
Manas Kumar Kundu and
Sudip Barman*
School of Chemical Sciences, National Institute of Science Education and Research (NISER), Bhubaneswar, Orissa - 751 005, India. E-mail: sbarman@niser.ac.in; Fax: +91 (674)2304070; Tel: +91 (674)2304061
First published on 5th November 2013
Abstract
Carbon quantum dots (CQDs) and two dimensional (2D) graphene sheets were prepared from formic acid by microwave mediated thermal method. Microwave irradiation followed by thermal evaporation of formic acid produces fluorescent CQDs. The fluorescence emission of CQDs in water can be tuned between 310 and 820 nm by changing the excitation wavelengths. These quantum dots are found to be sensitive towards hydrogen peroxide in aqueous medium due to quenching of fluorescence. The large area graphene sheets are formed on a solid substrate due to self assembly and 2D growth of CQDs. Graphene sheets modified glassy carbon electrode showed fast electron transfer kinetics for Fe(CN)63−/4− couple. Moreover, these modified electrodes can be used as a highly sensitive and selective metal free, non-enzymatic electrochemical sensor for hydrogen peroxide. The low detection limit was found to be 300 nM.
Introduction
Since the discovery of the single plane layer of honeycomb network of sp2 hybridized carbon atoms, graphene by Andrew Geim et al.1 it has attracted enormous amount of interest from the scientific community due to its unique properties.1,2 The interesting properties of graphene reported in the literature include ballistic electron transport, integer and fractional quantum hall effect, extremely high carrier mobility and ability to sustain very high current densities. Thus, it is considered as a next-generation material for nano-electronics, spintronics, sensors, energy storage. However, the synthesis of large-area graphene on different substrate is hindering its applications. Several methods1–5 such as mechanical exfoliation of graphite, chemical exfoliation, synthesis from solid carbon sources, chemical vapor deposition (CVD) of hydrocarbons on metal substrates, sublimation of silicon from silicon carbide (SiC) wafers etc. are available to synthesize a single or few layers of graphene. Although mechanical exfoliation of graphite provides excellent quality graphene, it is not suitable for large scale production of graphene. The main difficulty in producing high quality of graphene from graphite is especially due to the high van der Waals energy adhering graphene layers to one another. The most popular solution based approach is the chemical exfoliation from graphite which involves chemical oxidation of graphite into graphite oxide, followed by exfoliation of graphite oxide in water to form graphene oxide by ultrasonication.3 Chemical oxidation induces defects3,6 into the sheets due to presence of oxygenated functional groups such as carboxyl, hydroxyl, epoxy etc. Although reduction of graphene oxide by different reducing agent leads to increasing the π-conjugation of graphene,6 the restoration of complete π-conjugation is not possible to achieve and hence it has low conductivity. Although chemical vapor deposition (CVD) produces high quality graphene on metal surfaces, there is a need for reliable method(s) for the production of high yield, high quality and large surface area graphene on silicon (Si) substrate. Moreover, compared to 2D graphene less attention has been paid to carbon quantum dots. They are more suitable for various applications as compared to small organic fluorescent molecules and inorganic semiconductor quantum dots, because of their high resistance to photo bleaching, low cytotoxicity and superior biocompatibility. Common methods7 for the production of carbon quantum dots include pyrolysis of citric acid, ethylene diamine tetraacetic acid salts, the hydrothermal synthesis from grass and graphene sheets. Highly sensitive and selective determination of hydrogen peroxide is important8 since it is the product of several biological enzyme-catalyzed reactions and also plays an active role in food, pharmaceutical, clinical, industrial, and environmental analyses. Several analytical methods9 such as titration, chromatographic, fluorometric, colorimetric, chemiluminescent, electrochemical methods are available for determination of H2O2. Among these methods, fluorescence and electrochemical methods for determination of H2O2 are the best methods because of their low costs, high sensitivity and portability.Herein, we report a low temperature solution based synthesis of carbon quantum dots and large area graphene sheets from formic acid by a simple microwave assisted thermal method. The carbon quantum dots were found to be an excellent fluorescence probe for determination of hydrogen peroxide. The graphene sheets modified glassy carbon electrode showed fast electron transfer kinetics for Fe(CN)63−/4− redox couple. In addition, these modified electrode can be used as highly sensitive and selective non-enzymatic, metal free electrochemical determination of H2O2.
Experimental
Synthesis of carbon quantum dots
Formic acid (HCOOH) was used as a precursor for microwave assisted synthesis of carbon quantum dots (CQDs). Formic acid (HPLC grade) was purchased from Merck (Darmstadt, Germany) and used as received without any further purification. In a typical synthesis procedure, 30 mL of formic acid was heated by a microwave synthesizer at 90 °C for 3 h. Then, the resulting solution was vacuum evaporated several times in a rotary evaporator at 120 °C to produce CQDs. The whole amount was dissolved in 20 mL of water and used as a stock solution. The dynamic light scattering measurements were performed for determining the size of CQDs in solution. It was found that hydrodynamic diameter (average size) of CQDs in aqueous solution was 6, 80, 300, 330 nm at 2, 4, 20, 60 minutes respectively after solution preparation (ESI,† Fig. S1). The average size of CQDs in aqueous solution increases with time and is also changed with dilution and ultrasonication. This suggests that CQDs in solutions are self assembling in solution and held together by weak attractive forces.Characterization
MAS-II microwave synthesizer from Sineo Microwave Chemistry Technology Company (Shanghai, China) was used for the synthesis of carbon quantum dots (CQDs). Hydrodynamic diameter of CQDs in solutions was measured at 25 °C by dynamic light scattering (DLS) using the Malvern Zetasizer Nano ZS instrument. The characterization of CQDs and 2D graphene sheets was done using tapping mode of AFM (Asylum MFP-3D Atomic Force Microscope) and transmission electron microscopy (TEM, JEOL JEM2010, operated at 200 kV and TEM, FEI, Technai G2). The AFM and TEM samples were prepared by drop casting a drop (10μL) aqueous solution on AFM disc and TEM grid, then evaporate the solution in air at ∼45 °C. SEM images of graphene on copper and silicon substrate were obtained using field-emission scanning electron microscope (FESEM) system (Carl Zeiss, Germany make, Model: ∑igma). The SEM samples were prepared by drop casting 30 μL aqueous solution on silicon (Si) substrate and copper (Cu) foil, then evaporate the solution in air at ∼45 °C. Powder X-ray diffraction (p-XRD) patterns were performed on a Bruker DAVINCI D8 ADVANCE diffractometer equipped with Cu Kα radiation (λ = 0.15406 nm). XPS measurements on the sample were done using a monochromatic Mg Kα X-ray source (XPS VG Microtech). XPS sample was prepared by drop casting few drops of aqueous solution of CQDs on silicon substrate and evaporate at ∼45 °C. UV-visible spectra were recorded using a UV-visible spectrophotometer (Varian Cary 100 Bio). Fluorescence spectroscopic studies of CQDs were carried out using a spectrofluorimeter (Perkin Elmer, LS 55). Time resolved fluorescence lifetime measurements were performed using a time-correlated single photon counting (TCSPC) spectrometer (Edinburgh, OB920). FT-IR measurements of CQDs were performed using a Perkin-Elmer Spectrum RXI FT-IR spectrophotometer. Electrochemical measurements were performed with a conventional three electrode cell using an electrochemical workstation (CH Instrument, Model: 1100A).Preparation of thin graphene sheets modified glassy carbon electrode
Glassy carbon (GC) electrode was polished first with 1.0, 0.1 and 0.05 μm alumina slurry on Buehler microcloth polishing cloth. After rinsing with distilled water, the electrode was sonicated in distilled water for about 10 min. 30 μL of this aqueous stock solution of CQDs was drop cast on the cleaned GC electrode and allowed to evaporate the solvent at ∼45 °C. Hence, GC electrode is coated with a film of graphene sheets.Results and discussion
The atomic force microscopic (AFM) measurements were used to measure the size and thickness of CQDs. Fig. 1a and b represent the AFM images of CQDs which give the topographic height (Fig. 1c and d). They are in the range between 0.3 and 2.0 nm and their average height is about 1.0 nm. The stock solution of CQDs was diluted 200 times before being used for AFM sample preparation. A transmission electron microscopy (TEM) image of CQDs is shown in Fig. 1e. Their size varies ranging from 2–18 nm. In addition to these CQDs, large area sheets were also formed which will be discussed later. The AFM and TEM samples were prepared by drop casting a drop (10 μL) aqueous solution on AFM disc and TEM grid, then evaporate the solution in air. | ||
| Fig. 1 (a) and (b) AFM images of CQDs. (c) and (d) are the AFM line profile along the line AB and CD in the images (a) and (b) respectively. (e) TEM images of CQDs. | ||
The optical properties of CQDs were studied in their aqueous as well as non aqueous solutions. Fig. 2a displays the UV-visible and emission spectra of CQDs in aqueous solution. The absorption peak due to π → π* transition for aromatic sp2 domain appeared at ∼230 nm.7 In addition, an absorption peak centered at 276 nm was also observed in the UV-visible spectrum. Two emission peaks at 310 and 375 nm appeared when CQDs are excited at 276 nm. Most importantly, these fluorescence emissions were found to be dependent on the excitation wavelengths. When CQDs are excited at the different wavelength ranging from 200 to 650 nm, both emission peaks are shifted from 310 to 820 nm and fluorescence intensity gradually decreases with increase in excitation wavelength from 310 to 650 nm (Fig. 2b and c). The excitation wavelength dependent emission of graphene quantum dots,7 carbon nitride quantum dots,10 carbon dots11 are reported in literature. This is the first report to my knowledge that these CQDs exhibit deep ultra violet, visible as well as near infrared (NIR) fluorescence emission. However, there is a recent report of deep ultra violet emission12 of small sized graphene quantum dots. These CQDs owing to their NIR emission may find biological optical imaging and detection applications because of low tissue absorption and scattering effect in the NIR wavelength range (650–900 nm).13
Several groups suggested7,12 that the presence of conjugated structure, zigzag sites in graphene and emission trap on the surface is responsible for the fluorescence emission of CQDs. The fluorescence excitation (FLE) spectra of CQDs for 375 nm emission and fluorescence spectra are shown in Fig. 2d. The FLE spectrum exhibits three peaks at 230, 276 and 309 nm. If the CQDs are excited at 230, 276 and 309 nm, strong fluorescence emissions are observed. The FLE peaks at 230 and 276 nm correspond to the 230 and 276 nm absorption peaks respectively. The FLE spectra clearly suggests that the observed fluorescence emissions from CQDs may be directly correlated with the three new transitions at 230, 276 and 309 nm rather than commonly observed π → π* transition. According to Radovic and Bockrath,14 the carbene-like, with triplet ground state in zigzag sites where as carbyne like, with singlet ground state in armchair sites are most common in graphene. The electronic configuration for triplet ground state of carbene can be described as σ1 π1 and singlet carbene two non bonding electrons are paired in either σ or π orbital, leaving one orbital vacant. The carbene ground state spin multiplicity is related to energy difference (δE) between σ and π orbital; δE should be below 1.5 eV for carbene triplet ground state. In FLE spectra, the two electronic transitions at 230 nm (5.39 eV) and 276 nm (4.49 eV) can be described as a transition from the σ and π orbital (highest occupied molecular orbital, HOMO) to the lowest unoccupied molecular orbital (LUMO). The energy difference, δE was found to be 0.9 eV which is lower than the required value (<1.5 eV) for triplet state. This suggests that assignment of these two transitions is quite reasonable. The third transition at 310 nm in FLE spectrum may possibly be related to surface states. The schematic description of emissions as discussed above is depicted in Fig. S4.† In addition to excitation dependent emission, CQDs emission is strongly sensitive to the solvents, as shown in Fig. 2e. It shows that the variation of emission peak positions of CQDs with variation of solvents follows the trend methanol (315 nm), water (311 and 375 nm), cyclohexane (330 and 389 nm), benzene (339 and 404 nm), THF (355 and 405 nm). The excitation dependent emission spectra of CQDs in methanol, cyclohexane and benzene are shown in Fig. S2.† It shows that CQDs in methanol solution strong emission mainly in UV region while CQDs in cyclohexane, benzene, THF produce primarily blue emission. The fluorescence quantum yield (QY) of CQDs in water at the excitation wavelength of 280 nm was found to be 4.5% using quinine sulfate as a reference (see ESI† for details). The QY of CQDs is also sensitive to solvents and follow the trend methanol (1.1%), water (4.5%), cyclohexane (12%), THF (14%), benzene (17%). The fluorescence decay profile of CQDs in water is presented in Fig. 2f, which followed a tri-exponential behavior indicating the presence of three emitting species with three different lifetimes. Although the position of fluorescence emission wavelength and quantum yield of CQDs is strongly dependent on solvents, the average lifetime (τavg) of CQDs is independent of solvents (ESI†, Fig. S3 and Table S1). The QYs of CQDs as well as their average lifetime (τavg) are close to the reported values of carbon dots and graphene quantum dots.7,12 The average lifetime of CQDs is ∼6.0 ns indicating that CQDs are suitable for biological as well as optoelectronic applications. Apart from excitation and solvent dependent fluorescence behavior of CQDs, fluorescence intensity of CQDs in aqueous medium is sensitive to the pH of the medium. Fig. 3a shows the fluorescence intensity is lowest in acidic pH and highest in basic medium. The possible explanation of quenching of fluorescence of CQDs in acidic medium is probably due to the protonation of zigzag sites of graphene in acidic medium to form a non-fluorescent complex.
The variation of fluorescence emission intensity of CQDs with the added hydrogen peroxide is shown in Fig. 3b and c. The fluorescence response of CQDs in presence of hydrogen peroxide and different ions suggested that the emission of CQDs only sensitive towards hydrogen peroxide (Fig. 3d). No significant spectral change of CQDs was observed in the presence of different ions (such as Hg2+, Pb2+, Cu2+, Zn2+, Ni2+, ClO− and glucose). The calibration plot (Fig. 3c) for the determination of H2O2 is linear over the range from 10 nM to 5 μM. The detection limit of H2O2, calculated from the Fig. 3c was ∼700 nM. Thus, CQDs can be used as fluorescence probe for selective and sensitive determination of H2O2 due to quenching of their fluorescence.
The 2D graphene thin sheets are formed due to evaporation induced self assembly of CQDs and their growth on a solid substrate. The TEM images of graphene sheets are shown in the Fig. 4a, c and d. The size of sheets varies from few hundreds of nanometer to few microns. Fig. 4b presents a representative selected area electron diffraction (SAED) pattern from graphene sheets in Fig. 4a. The crystal planes corresponding to d-spacing of 2.139, 1.233, 1.059 Å, calculated from the ring of SAED image, were indexed as (100), (110), (201) respectively. This is in agreement with the reported results for single or bi-layer of graphene.15 The high resolution TEM (HRTEM) images of graphene sheets are shown in Fig. 4e, S5b and c.† The crystalline lattice of graphene is clearly seen in the HRTEM images. The sheets are multi layered graphene sheets consisting of 20–40 graphene layers as shown in Fig. S5c.† The inter planner distance was measured from lattice fringes to be 0.35 nm which is consistent with the spacing of the (002) planes of hexagonal graphite. A closer look of TEM images (Fig. 4a, c and d) reveals the transparent and several stacked layers of 2D graphene sheets indicating flat morphology of these sheets.
The folded graphene sheet can also be seen in Fig. S5a.† The energy dispersive X-ray spectroscopic (EDX) measurement was carried out to analyze the chemical composition of graphene sheets. Fig. S5d† shows EDX spectrum of graphene sheets indicating the presence of mostly carbon with small amount of oxygen and copper. Thus, TEM measurements proved that evaporation induced self assembly of CQDs and growth in two dimensions lead to the formation of 2D multi layered graphene sheets. In addition, graphite nanoparticles (ESI,† Fig. S6a) are produced when the stock solution was diluted 100 times with water before being used for TEM sample preparation. The calculated inter planner distances of 3.2, 2.12, 1.80, 1.62, 1.23 Å from SAED image (ESI,† Fig. S6b) correspond to the (002), (100), (102), (004) and (110) planes respectively of hexagonal graphite (PDF-00-041-1487). CQDs were aggregated on dilution and thereby graphite nanoparticles were formed. The powder X-ray diffraction pattern of graphene/graphite on quartz substrate is shown in Fig. S7.† A sharp intense peak at 26.80 (2θ) with interlayer spacing of 3.3 Å is indexed as 002 reflection of graphitic sheets. In addition, two peaks appeared at lower 2θ values at 20.05 (d = 4.98 Å) and 9.2 (d = 9.7 Å). The oxidation3,16 of graphite causes the shift of (002) reflection peak to lower values and the increase in d-spacing is due to the intercalation of water molecules and the formation of different oxygen functionalities in graphite oxide. The interlayer spacing of 9.7 Å is close to reported d-value for graphene oxide.17 Increased d-spacing (9.7 and 4.98 Å) can be attributed to the intercalation of water/the presence oxygen functionalities in graphene sheets.
The field emission electron microscopy (FESEM) images of graphene sheets are presented in the Fig. 5a–d. Different morphologies of graphene were observed during SEM measurements. The thin, flat graphene sheets were formed on copper foil (Fig. 5a) which is in accordance with the TEM observations. As shown in Fig. 5b, the graphene flakes are vertically oriented with respect to copper substrate. Fig. 5d is the SEM image of graphene film on silicon substrate showing the wrinkle and protrusion morphologies of graphene. We have not noticed any charging during SEM imaging indicting that sheets are electrically conductive. Fig. 6a and c present AFM image of graphitic film on mica indicating few-micron sized continuous graphene film. Fig. 6a and line profile along AB (Fig. 6b) suggest that several sheets are self assembled on mica disc and their thickness varies from 3 to 10 nm. Thus, each sheet consists of 10–30 graphene layers, which is in agreement with HRTEM results. Based on DLS, TEM, AFM, SEM studies a proposed growth mechanism of graphene sheets is shown schematically in Fig. S8.†
 | ||
| Fig. 6 (a) AFM image of graphene film showing the several sheets. (b) Line profile along the AB. (c) AFM image micron sized graphene film. | ||
X-ray photo electron spectroscopy (XPS) was used to examine the chemical environment around carbon in graphene sheets. Fig. 7a shows the XPS survey scan of graphene sheets indicating the presence of carbon and oxygen with no other impurities. The C1s XPS spectra of graphene sheets was deconvoluted to three different components (Fig. 7b). The binding energy of 284.5 eV is attributed to sp2 hybridized carbon in aromatic rings18 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C). The peaks at 285.9 eV and 287.56 eV can be assigned to carbon bonded to hydroxyl (C–OH) and carbonyl (C
C). The peaks at 285.9 eV and 287.56 eV can be assigned to carbon bonded to hydroxyl (C–OH) and carbonyl (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) group18 respectively. Fig. 7c shows the Fourier transform infrared (FT-IR) spectrum of CQDs. The intense band at ∼1600 cm−1 corresponds to aromatic C
O) group18 respectively. Fig. 7c shows the Fourier transform infrared (FT-IR) spectrum of CQDs. The intense band at ∼1600 cm−1 corresponds to aromatic C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretch whereas bands at 1088 cm−1 can be assigned to C–O stretch.19 The O–H deformation peak appeared at 1380 cm−1. The two peaks at 2925 and 2854 cm−1 correspond to an asymmetric and symmetric stretch of CH2 group whereas the broad band at 3420 cm−1 can be attributed to O–H stretch. A peak at 800 cm−1 can be assigned to the C–H out of plane bending vibration and the low intense peaks between 580 cm−1 to 800 cm−1 can be attributed to the vibration of the C–H bonds in benzene rings.19 Thus, microscopic and spectroscopic studies confirm the successful growth of 2D graphene sheets.
C stretch whereas bands at 1088 cm−1 can be assigned to C–O stretch.19 The O–H deformation peak appeared at 1380 cm−1. The two peaks at 2925 and 2854 cm−1 correspond to an asymmetric and symmetric stretch of CH2 group whereas the broad band at 3420 cm−1 can be attributed to O–H stretch. A peak at 800 cm−1 can be assigned to the C–H out of plane bending vibration and the low intense peaks between 580 cm−1 to 800 cm−1 can be attributed to the vibration of the C–H bonds in benzene rings.19 Thus, microscopic and spectroscopic studies confirm the successful growth of 2D graphene sheets.
 | ||
| Fig. 7 (a) XPS survey scan and (b) C1s XPS spectrum of graphene on silicon substrate. (c) FT-IR spectra of CQDs. | ||
Another aspect of this work is the electron transfer kinetics for electrochemical reaction of Fe(CN)63−/4− at graphene modified electrode/solution interface using aqueous solution containing Fe(CN)63−/4− and highly sensitive non-enzymatic detection of hydrogen peroxide. It is well known that the electrochemical reaction of Fe(CN)63−/4− is sensitive to surface,20 but less significant effect on oxygen functionalities in carbon based electrode.21 Fig. 8a shows a comparison of cyclic voltammetric (CV) responses in 1.0 M KCl solution containing potassium ferrocyanide at bare glassy carbon (GC) electrode and graphene sheets modified glassy carbon (GS/GC) electrode. The CV at bare GC electrode showed a redox peak with a peak potential difference (ΔEp) of 68 mV indicating a quasi-reversible redox reaction. However, the CV obtained using GS/GC electrode exhibited a redox peak with ΔEp of ∼60 mV under similar experimental condition. In addition, enhancement of 1.4 times peak currents are also observed when electrode is modified with graphene sheets. The ΔEp is ∼60 mV with enhancement of peak currents suggesting fast electron-transfer kinetics for Fe(CN)63−/4− redox couple. The CVs obtained with various scan rate ranging from 10 mV to 900 mV at GS/GC electrode using 1.0 M KCl solution containing 1 mM Fe(CN)63−/4− are shown in Fig. 8b. As shown in Fig. 8c, the anodic and cathodic peak currents increases linearly with the square root of the scan speed suggesting that reaction is predominantly diffusion controlled. Similar experiments were carried out using several GS/GC electrodes and it was observed that ΔEp values were always in the range 60–64 mV. This slight variation of ΔEp value is probably due to the different quality of graphene sheets that are formed on GC electrode surface in each case. The fast electron transfer in graphene and better electrochemical behaviour were reported22 due to the presence sharp edge plane in graphene. The sharp edges of graphene sheets can easily be seen in TEM, SEM, AFM images (Fig. 4–6). Thus, we can conclude that fast electron transfer kinetics of these graphene sheets can be attributed to the presence sharp edges of graphene sheets.
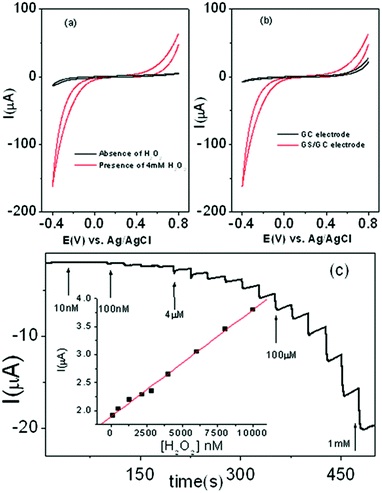
The electrocatalytic property of graphene modified GC electrode towards the electrochemical reduction and oxidation of H2O2 was studied using cyclic voltammetry. Fig. 9a shows cyclic voltamograms at GS/GC electrode in absence and presence of 4 mM H2O2 in 0.1 M PBS buffer solution. A small background current was observed in the blank 0.1 M PBS solution at GS/GC electrode where as a drastic increase of electrocatalytic current was observed in 0.1 M PBS solution containing 4 mM H2O2. Fig. 9b represents the comparison of the voltametric response of 4 mM H2O2 in 0.1 M PBS solution at bare GC electrode and GS/GC electrode. This suggests the superior electrocatalytic activity at GS/GC electrode toward H2O2. Fig. 9c shows a typical amperometric current-time response at GS/GC electrode for successive addition of H2O2 into stirring 0.1 M PBS buffer solution at an applied potential of −0.4 V. As shown in calibration plot (ESI†, Fig. S9a), there are two regions ranging 100 nM to 10 μM and 10 μM to 1 mM, where a linear relation of current with concentration of H2O2 was observed. From the slope of calibration plot as shown in the inset of Fig. 9c, the low detection limit at GS/GC electrode to H2O2 was measured to be 300 nM at signal-to-noise ratio of 3 (R = 0.999). This experimental detection limit of GS/GC electrode for H2O2 detection is comparable to the reported graphene based electrochemical H2O2 sensor.23 However, the detection limit of ∼50 nM for metal nanoparticle or biomolecule funationalized graphene bio-sensors24 was reported recently. The enhanced electrocatalytic behavior of graphene sheets is probably due to their fast electron transfer of graphene sheets owing to the presence of sharp edge planes in the graphene sheets. The interference of other biomolecules such as dopamine (DA), ascorbic acid (AA), D-glucose, L-glycine, L-cysteine, L-tyrosine, L-tryptophan on biosensor response were examined. As shown in Fig. S9b,† the addition of 10 μM of H2O2 causes a significant increase of the amperometric response at the applied potential of −0.4 V, but addition of similar concentration of AA, DA, D-glucose, L-glycine, L-cysteine, L-tyrosine, L-tryptophan did not cause interference the amperometric response of H2O2. The reproducibility of the current response for ten different electrodes was examined in 0.1 M solution containing 4 mM H2O2 by measuring cyclic voltammetric scans. The relative standard deviation (RSD) was 8% indicating that graphene modified electrode had good reproducibility.
Conclusions
In conclusion, we have demonstrated relatively low temperature (∼120 °C) synthesis of fluorescent carbon quantum dots (CQDs) and 2D graphene from formic acid. These CQDs exhibit deep ultraviolet, visible as well as near infrared emission in aqueous solution. We have shown the application CQDs as sensitive fluorescence sensors for H2O2 in aqueous media. The micron sized multi layer graphene sheets are formed from CQDs on a solid substrate by their self assembly and growth in two dimensions. The graphene sheets modified glassy carbon electrode exhibits fast electron transfer kinetics for Fe(CN)63−/4− redox couple indicating the possibility of a graphene based electrode for electrochemical applications. We have also demonstrated that graphene sheets modified glassy carbon electrode can be used for highly sensitive and selective non-enzymatic determination of H2O2.Acknowledgements
S.B. thanks DST, Govt. of India for financial assistance and Institute of Physics, Bhubaneswar, India for XPS, TEM, and AFM measurements. We would also thank Mr T. Basu, Mr A. Rath and Mr S. K. Chowdhury for their help with AFM, TEM and XPS measurements. M.S. and T.B. contributed equally to this work.References
- K. S. Novoselov, A. K. Geim, S. V. Morozov, D. Jiang, Y. Zhang, S. V. Dubonos, I. V. Grigorieva and A. A. Firsov, Science, 2004, 306, 666 CrossRef CAS PubMed.
- A. K. Geim and K. S. Novoselov, Nat. Mater., 2007, 6, 183 CrossRef CAS PubMed; A. K. Geim, Science, 2009, 324, 1530 CrossRef PubMed; J. H. Seol, I. Jo, A. L. Moore, L. Lindsay, Z. H. Aitken, M. T. Pettes, X. Li, Z. Yao, R. Huang, D. Broido, N. Mingo, R. S. Ruoff and L. Shi, Science, 2010, 328, 213 CrossRef PubMed; Y. B. Zhang, Y. W. Tan, H. L. Stormer and P. Kim, Nature, 2005, 438, 201 CrossRef PubMed; C. N. R. Rao, A. K. Sood, K. S. Subrahmanyam and A. Govindaraj, Angew. Chem., Int. Ed., 2009, 48, 7752 CrossRef PubMed; K. I. Bolotin, F. Ghahari, M. D. Shulman, H. L. Stormer and P. Kim, Nature, 2009, 462, 196 CrossRef PubMed.
- S. Park and R. S. Ruoff, Nat. Nanotechnol., 2009, 4, 217 CrossRef CAS PubMed.
- Y. Hernandez, V. Nicolosi, M. Lotya, F. M. Blighe, Z. Sun, S. De, I. T. McGovern, B. Holland, M. Byrne, Y. K. Gun'ko, J. J. Boland, P. Niraj, G. Duesberg, S. Krishnamurthy, R. Goodhue, J. Hutchison, V. Scardaci, A. C. Ferrari and J. N. Coleman, Nat. Nanotechnol., 2008, 3, 563 CrossRef CAS PubMed; Z. Sun, Z. Yan, J. Yao, E. Beitler, Y. Zhu and J. M. Tour, Nature, 2010, 468, 549 CrossRef PubMed.
- M. Wang, L. Fu, L. Gan, C. Zhang, M. Rümmeli, A. Bachmatiuk, K. Huang, Y. Fang and Z. Liu, Sci. Rep., 2013, 3, 1238 Search PubMed; C. Berger, Z. Song, X. Li, X. Wu, N. Brown, C. Naud, D. Mayou, T. Li, J. Hass, A. N. Marchenkov, E. H. Conrad, P. N. First and W. A. de Heer, Science, 2006, 312, 1191 CrossRef CAS PubMed.
- S. Stankovich, D. A. Dikin, R. D. Piner, K. A. Kohlhaas, A. Kleinhammes, Y. Jia, Y. Wu, S. T. Nguyen and R. S. Ruoff, Carbon, 2007, 45, 1558 CrossRef CAS PubMed.
- M. J. Krysmann, A. Kelarakis, P. Dallas and E. P. Giannelis, J. Am. Chem. Soc., 2012, 134, 747 CrossRef CAS PubMed; D. Pan, J. Zhang, Z. Li and M. Wu, Chem. Commun., 2010, 46, 3681 RSC; D. Pan, J. Zhang, Z. Li and M. Wu, Adv. Mater., 2010, 22, 734 CrossRef PubMed; S. Liu, J. Tian, L. Wang, Y. Luo, J. Zhai and X. Sun, J. Mater. Chem., 2011, 21, 11726 RSC; S. Liu, J. Tian, L. Wang, Y. Zhang, X. Qin, Y. Luo, A. M. Asiri, A. O. Al-Youbi and X. Sun, Adv. Mater., 2012, 24, 2037 CrossRef PubMed.
- J. Wang, Chem. Rev., 2008, 108, 814 CrossRef CAS PubMed; T. M. Schreier, J. J. Rach and G. E. Howe, Aquaculture, 1996, 140, 323 CrossRef.
- V. Klassen, D. Marchington and H. C. E. McGovan, Anal. Chem., 1994, 66, 2921 CrossRef CAS; M. C. Y. Chang, A. Pralle, E. Y. Isacoff and C. J. Chang, J. Am. Chem. Soc., 2004, 126, 15392 CrossRef PubMed; G. Shi, J. Lu, F. Xu, H. G. Zhou, L. Jin and J. Jin, Anal. Chim. Acta, 2000, 413, 131 CrossRef; D. W. King, W. J. Cooper, S. A. Rusak, B. M. Peake, J. J. Kiddle, D. W. O'Sullivan, M. L. Melamed, C. R. Morgan and S. M. Theberge, Anal. Chem., 2007, 79, 4169 CrossRef PubMed; W. Chen, S. Cai, Q. Ren and Y. Zhao, Analyst, 2012, 137, 49 RSC; Q. Chang, L. Zhu, G. Jiang and H. Tang, Anal. Bioanal. Chem., 2009, 395, 2377 CrossRef PubMed; S. Liu, J. Tian, L. Wang, Y. Luo and X. Sun, RSC Adv., 2012, 2, 411 RSC.
- S. Barman and M. Sadhukhan, J. Mater. Chem., 2012, 22, 21832 RSC.
- C. W. Lai, Y. H. Hsiao, Y. K. Peng and P. T. Chou, J. Mater. Chem., 2012, 22, 14403 RSC.
- L. Tang, R. Ji, X. Cao, J. Lin, H. Jiang, X. Li, K. S. Teng, C. M. Luk, S. Zeng, J. Hao and S. P. Lau, ACS Nano, 2012, 6, 5102 CrossRef CAS PubMed.
- J. M. Klostranec and W. C. W. Chan, Adv. Mater., 2006, 18, 1953 CrossRef CAS.
- L. R. Radovic and B. Bockrath, J. Am. Chem. Soc., 2005, 127, 5917 CrossRef CAS PubMed.
- J. C. Meyer, A. K. Geim, M. I. Katsnelson, K. S. Novoselov, T. J. Booth and S. Roth, Nature, 2007, 446, 60 CrossRef CAS PubMed.
- T. Kuila, S. Bose, P. Khanra, A. K. Mishra, N. H. Kim and J. H. Lee, Carbon, 2012, 50, 914 CrossRef CAS PubMed.
- D. C. Marcano, D. V. Kosynkin, J. B. Berlin, A. Sinitskii, Z. Sun, A. Slesarev, L. B. Alemany, W. Lu and J. M. Tour, ACS Nano, 2010, 4, 4806 CrossRef CAS PubMed.
- D. Chen, H. Feng and J. Li, Chem. Rev., 2012, 112, 6027 CrossRef CAS PubMed.
- C. W. Tsai, M. H. Tu, C. J. Chen, T. F. Hung, R. S. Liu, W. R. Liu, M. Y. Lo, Y. M. Peng, L. Zhang, J. Zhang, D. S. Shy and X. K. Xing, RSC Adv., 2011, 1, 1349 RSC; N. A. Kumar, H. J. Choi, Y. R. Shin, D. W. Chang, L. Dai and J. B. Baek, ACS Nano, 2012, 6, 1715 CrossRef CAS PubMed.
- P. Chen and R. L. McCreery, Anal. Chem., 1996, 68, 3958 CrossRef CAS.
- N. G. Shang, P. Papakonstantinou, M. McMullan, M. Chu, A. Stamboulis, A. Potenza, S. S. Dhesi and H. Marchetto, Adv. Funct. Mater., 2008, 18, 3506 CrossRef CAS.
- M. Zhou, Y. Zhai and S. Dong, Anal. Chem., 2009, 81, 5603 CrossRef CAS PubMed; K. Zhou, Y. Zhu, X. Yang, J. Luo, C. Li and S. Luan, Electrochim. Acta, 2010, 55, 3055 CrossRef PubMed; Y. Fang, D. Zhang, X. Qin, Z. Miao, S. Takahashi, J. Anzai and Q. Chen, Electrochim. Acta, 2012, 70, 266 CrossRef PubMed.
- Y. Shao, J. Wang, H. Wu, J. Liu, I. A. Aksay and Y. Lin, Electroanalysis, 2010, 22, 1027 CrossRef CAS.
- X. Cao, Z. Zeng, W. Shi, P. Yep, Q. Yan and H. Zhang, Small, 2013, 9, 1703 CrossRef CAS PubMed; F. Xi, D. Zhao, X. Wang and P. Chen, Electrochem. Commun., 2013, 26, 81 CrossRef PubMed; D. Chen, H. Feng and J. Li, Chem. Rev., 2012, 112, 6027 CrossRef PubMed; B. Yu, J. Feng, S. Liu and T. Zhang, RSC Adv., 2013, 3, 14303 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Details of the experimental techniques, quantum yield measurements, fluorescence lifetimes of CQDs. Additional Table S1 and Figures (Fig. S1–S9). See DOI: 10.1039/c3ra46050a |
| This journal is © The Royal Society of Chemistry 2014 |