Electrochemical properties of poly(3,4-ethylenedioxythiophene) grown on Pt(111) in imidazolium ionic liquids
Abstract
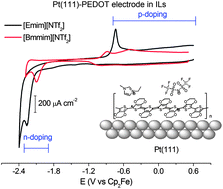
Poly(3,4-ethylenedioxythiophene) (PEDOT) was galvanostatically synthesized and studied on a Pt(111) electrode in the following ionic liquids: 1-ethyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide ([Emim][NTf2]), 1-ethyl-2,3-dimethylimidazolium bis(trifluoromethylsulfonyl)imide ([Emmim][NTf2]), 1-butyl-3-methylimidazolium trifluoromethanesulfonate ([Bmim][OTf]) and 1-butyl-2,3-methylimidazolium bis(trifluoromethylsulfonyl)imide ([Bmmim][NTf2]) by cyclic voltammetry, chronoamperometry and electrochemical impedance spectroscopy. The thin films prepared with this method and cycled in these ionic liquids show high n-doping currents and high rates of ion exchange during redox switching compared to those obtained in molecular solvents.

 Please wait while we load your content...
Please wait while we load your content...