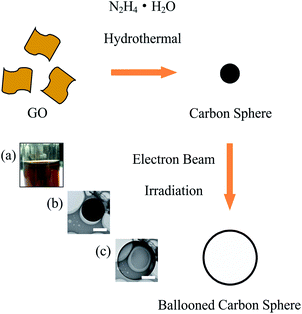
Electron beam “ballooned” carbon sphere derived from graphene oxide by a hydrazine assisted hydrothermal method†
Yaming Wang*a,
Jianyun Caoa,
Jiancun Raoa,
Xiaoxiao Lub,
Ping Xiaob,
Yu Zhoua,
Dechang Jiaa and
Jia-Hu Ouyanga
aInstitute for Advanced Ceramics, Harbin Institute of Technology, Harbin 150001, China. E-mail: wangyaming@hit.edu.cn; Fax: +86-451-86413922; Tel: +86-451-86413910
bMaterials Science Centre, School of Materials, University of Manchester, Manchester M1 7HS, UK
First published on 18th December 2013
Abstract
Self-assembled carbon spheres are synthesized by a hydrazine assisted hydrothermal method using graphene oxide as precursor. The as-prepared carbon spheres show dramatic shape changes (ballooning) in response to the electron beam as an external trigger.
Molecular assemblies with controllable shape changes in response to an external trigger will be crucial to manufacture nanometer-scale devices.1 Although many efforts have been devoted into the development of these “smart” systems, such as thermally responsive pulsating nanotubules,2 pH-induced “breathing” vesicles3 and photo-driven pulsating vesicles.4 It remains a great challenge to create new stimulus-responsive systems responding to various external triggers. Graphene oxide (GO) sheets, which could be thought as “soft” two-dimensional macromolecules, have been used as building blocks to assemble different interesting structures.5,6 Since it was first demonstrated that hydrothermal method can be used as an effective way to handle GO,7 enormous interesting graphene based materials have been derived from GO through hydrothermal process.8–15 For example, by tuning the chemical environments (such as pH, salinity and temperature), GO could be assembled to macroscopic structures,10,11 cut into graphene quantum dots,12,13 or doped with hetero atoms.14,15 However, there is no reports about the possibility of using GO or GO derivates to develop stimulus-responsive systems so far. Herein, we report that self-assembled carbon spheres derived from GO by a hydrazine assisted hydrothermal method could undergo dramatic shape changes (ballooning) in response to the electron beam irradiation as external trigger.
Fig. 1 illustrates the process for fabricating carbon spheres and the consequent electron beam induced ballooning. GO aqueous suspension (Fig. 1a) was prepared by sonication of graphite oxide in distilled water. The as-prepared GO aqueous suspension (1 mg mL−1) was directly mixed with 50% hydrazine hydrate aqueous solution with a volume ration of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. After being homogenized by stirring for 5 minutes, a total of 30 mL GO/hydrazine hydrate mixed aqueous suspension was transferred to a 40 mL Teflon-lined autoclave and heated at 160 °C for 10 h to generate solid carbon spheres. Then the self-assembled carbon spheres were collected by filtration, washed with distilled water for 3 times, and then redispersed in distilled water with mild sonication. The sample for electron beam irradiation treatment under a transmission electron microscope (TEM) was prepared by drop-drying the samples from the diluted aqueous suspension onto copper grids coated with holey carbon film. Samples for other characterizations were simply prepared by drying the filtration products in an oven at 80 °C for 10 h.
1. After being homogenized by stirring for 5 minutes, a total of 30 mL GO/hydrazine hydrate mixed aqueous suspension was transferred to a 40 mL Teflon-lined autoclave and heated at 160 °C for 10 h to generate solid carbon spheres. Then the self-assembled carbon spheres were collected by filtration, washed with distilled water for 3 times, and then redispersed in distilled water with mild sonication. The sample for electron beam irradiation treatment under a transmission electron microscope (TEM) was prepared by drop-drying the samples from the diluted aqueous suspension onto copper grids coated with holey carbon film. Samples for other characterizations were simply prepared by drying the filtration products in an oven at 80 °C for 10 h.
The in situ TEM experiment reveals the whole process of electron beam induced shape changes of the as-prepared carbon sphere (Movie S1, ESI†). Fig. 2 shows a series of images taken from the movie, in the first image (0 s), a pristine solid carbon sphere derived from GO with a diameter of 1.8 μm is shown. A slight volume shrinkage can be observed after electron beam irradiation for 10 s. And then, in the following frames (13–20 s), the carbon sphere experiences remarkable volume expansion until the diameter reaches about 3 μm. At the same time, the carbon sphere forms a unique hollow structure inside, with the sphere shell become thinner and thinner during the volume expansion. This process is like blowing balloons in our daily life. When the irradiation time exceeds 20 s, the as-formed hollow carbon sphere reaches its stable state, maintaining the same size during the following irradiation time (25–30 s).
 | ||
| Fig. 2 In situ TEM image series (captured from Movie S1, ESI†) depicting the whole process of electron beam induced shape changes of carbon sphere. | ||
The same stimulus-responsive phenomenon can also be reproduced by using a scanning electron microscope (SEM). Fig. 3 shows the over view SEM images of the pristine carbon spheres and the same area after electron beam irradiation for 100 s, all of the solid carbon spheres have been “ballooned” to hollow carbon spheres after irradiation, some show remarkable volume expansion (marked by white arrow).
Fig. 4 shows TEM images of the “ballooned” hollowed carbon spheres (Fig. 4a and c), and the corresponding high resolution TEM (HRTEM) images of the spheres' shells (Fig. 4b and d). The HRTEM images of the shells indicate that the shell is about 30 nm in thickness and exhibits two types of structures: (1) all amorphous structure (Fig. 4b); (2) amorphous inner layer with crystalline graphite outer layer (Fig. 4d). The crystalline graphite outer layer is about 5 nm in thickness, and there is no obvious interface between the outer layer and amorphous inner layer.
 | ||
| Fig. 4 TEM images of ballooned carbon spheres (a), (c) and HRTEM image of the corresponding sphere shells (b), and (d). | ||
The surface chemistry of the carbon sphere was analyzed by X-ray photoelectron spectroscopy (XPS). Fig. 5a shows the C1s spectrum of GO and carbon spheres, which revealing that most of oxygen functional groups has been removed after hydrazine assisted hydrothermal treatment, (the oxygen content decreased from 28.0 to 16.7 at.%). Raman spectroscopy is a practical method for characterizing graphitic and disordered carbons, in this case, particularly useful to study the amorphous carbon sphere. Fig. 5b shows the Raman spectrum of GO and the as-prepared carbon sphere. The typical features in the Raman spectra are the G band at 1580 cm−1 and D band at 1358 cm−1. The G band is usually assigned to the E2g phonon of C sp2 atoms, while the D band is the breathing mode of κ-point phonons of A1g symmetry, a prominent D band is an indication of disorder in the graphitic structure.17 The ID/IG ratio increased from 0.83 to 1.14 after the hydrazine assisted hydrothermal treatment, indicating that the as-prepared carbon sphere shows more disordered graphitic structure than GO, despite the removal of most of the oxygen functional groups.
 | ||
| Fig. 5 (a) C1s XPS spectrum of GO and carbon sphere. (b) Raman spectrum of GO and the as-prepared carbon sphere. | ||
As it has been demonstrated that GO layers could self-organize into a jelly-fish like structure by a combined hydrothermal method in the presence of hydrazine and ammonia,16 or aggregate and scroll up giving rise to other graphitic nanoforms with hydrothermal process in various pH conditions.17 We believe that the formation of pristine solid carbon sphere could be attributed to the unique hydrothermal environment and the strong reducibility of hydrazine. To confirm this, we replaced hydrazine with sodium borohydride which is another widely used reductant in GO research, and many carbon spheres were formed after hydrothermal treatment (see Fig. S1, ESI†). GO sheets could be reduced to reduced GO sheets (r-GO) quickly under hydrothermal environment with the existence of excessive hydrazine reductant. The hydrophobic r-GO sheets tend to enfold around the hetero atoms or defects, which act as nucleation sites, to form r-GO aggregates particles (Fig. S2† shows the TEM images of suspecting r-GO aggregates particles). With the increasing time of hydrothermal treatment, the firstly enfolded carbon layers would decompose to form an amorphous sphere core, for some spheres, the later enfolded outer layer could still maintain the crystalline graphite structure while some not. This enfolding-decomposition mechanism assumption is consistent with the unique shell structure of amorphous inner layer with crystalline graphite outer layer obtained from HRTEM results (Fig. 4). The whole process is also energetically favorable, as it can minimize the interfacial energy of the graphene sheets.
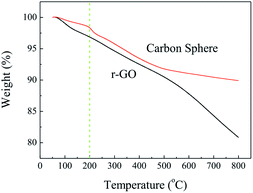
It is well known that oxidized graphene could generate lots of gas products once encountered with high temperature, such as thermal shock exfoliation,18 laser beam reduction,19 etc. Despite the hydrazine assisted hydrothermal reduction, the as-prepared carbon spheres still contains 16.7 at.% of oxygen. So, a reasonable mechanism for the electron beam induced shape changes of carbon spheres is that the electron beam will generate a localized heat during the irradiation process. Once the temperature reaches a certain value, the remaining oxygen groups will decompose and generate certain amount of gas products inside the carbon sphere and balloon the carbon sphere. Fig. 6 shows the thermogravimetry (TG) analysis of r-GO and as-prepared carbon spheres in argon flow. It is worth noticing that there is a fast mass loss around 200 °C for carbon spheres when comparing with r-GO. So we annealed the as-prepared carbon spheres at 200 °C for 2 h. Fig. 7 shows the SEM images of the annealed carbon spheres. All of the carbon spheres are broken after annealing, and the remaining carbon spheres' shell exhibits a pit-like surface morphology. The remaining shell is composed of wrinkled graphene sheets which can be observed from the high magnification SEM image (Fig. 7b). These results indicate that heating will lead to dramatic shape transformation of the as-prepared carbon spheres, thus confirming the assumption of electron beam heating function. However, due to that the annealing in a furnace is relatively uncontrollable (overall heating, invisible) when compared with electron beam heating in an electron microscopy (localized heating, visible), we can only get broken carbon spheres through annealing. The detailed mechanism for both the formation of pristine carbon sphere and electron induced shape change are still not well clear, which need further study.
 | ||
| Fig. 7 SEM images of broken carbon spheres after annealed at 200 °C for 2 h: (a) low magnification; (b) high magnification. | ||
In conclusion, we have shown that self-assembled carbon spheres derived from GO by a hydrazine assisted hydrothermal method could undergo dramatic shape changes (ballooning) in response to the electron beam irradiation as external trigger. In situ TEM characterization clearly show the whole process of the stimulus-responsive process of electron beam induced “ballooning” of carbon spheres. This research will provide a new route to develop stimulus-responsive systems, thus it generated a new method to manufacture nanometer-scale devices. What's more, this research also enlarged the application scope of graphene based materials, and will be helpful for development of novel smart materials based on graphene.
The partial supports from the NSFC grant no. 51371071 and 51021002, National Basic Science Research Program (2012CB933900), the Fundamental Research Funds for the Central Universities (HIT. BRETIII.201202) and the program for New Century Excellent Talents in University of China (NCET-08-0166) are gratefully acknowledged.
Notes and references
- J. C. M. van Hest, Nature, 2009, 461, 45–47 CrossRef CAS PubMed.
- Z. Huang, S. K. Kang, M. Banno, T. Yamaguchi, D. Lee, C. Seok, E. Yashima and M. Lee, Science, 2012, 337, 1521–1526 CrossRef CAS PubMed.
- S. Yu, T. Azzam, I. Rouiller and A. Eisenberg, J. Am. Chem. Soc., 2009, 131, 10557–10566 CrossRef CAS PubMed.
- J. Hu, H. Yu, L. H. Gan and X. Hu, Soft Matter, 2011, 7, 11345–11350 RSC.
- Y. Xu and G. Shi, J. Mater. Chem., 2011, 21, 3311–3323 RSC.
- C. Cheng and D. Li, Adv. Mater., 2013, 25, 13–20 CrossRef CAS PubMed.
- Y. Zhou, Q. Bao, L. A. L. Tang, Y. Zhong and K. P. Loh, Chem. Mat., 2009, 21, 2950–2956 CrossRef CAS.
- L. Qiu, X. Zhang, W. Yang, Y. Wang, G. P. Simon and D. Li, Chem. Commun., 2011, 47, 5810–5812 RSC.
- J. Cao, Y. Wang, P. Xiao, Y. Chen, Y. Zhou, J. H. Ouyang and D. Jia, Carbon, 2013, 56, 389–391 CrossRef CAS PubMed.
- Y. Xu, K. Sheng, C. Li and G. Shi, ACS Nano, 2010, 4, 4324–4330 CrossRef CAS PubMed.
- Z. Dong, C. Jiang, H. Cheng, Y. Zhao, G. Shi, L. Jiang and L. Qu, Adv. Mater., 2012, 24, 1856–1861 CrossRef CAS PubMed.
- D. Pan, J. Zhang, Z. Li and M. Wu, Adv. Mater., 2010, 22, 734–738 CrossRef CAS PubMed.
- M. Li, W. Wu, W. Ren, H. M. Cheng, N. Tang, W. Zhong and Y. Du, Appl. Phys. Lett., 2012, 101, 103107 CrossRef PubMed.
- H. L. Guo, P. Su, X. Kang and S. K. Ning, J. Mater. Chem. A, 2013, 1, 2248–2255 CAS.
- L. Sun, L. Wang, C. Tian, T. Tan, Y. Xie, K. Shi, M. Li and H. Fu, RSC Adv., 2012, 2, 4498–4506 RSC.
- D. Long, W. Li, L. Ling, J. Miyawaki, I. Mochida and S. H. Yoon, Langmuir, 2010, 26, 16096–16102 CrossRef CAS PubMed.
- C. Bosch-Navarro, E. Coronado, C. Martí-Gastaldo, J. F. Sánchez-Royo and M. G. Gómez, Nanoscale, 2012, 4, 3977–3982 RSC.
- H. C. Schniepp, J. Li, M. J. McAllister, H. Sai, M. Herrera-Alonso, D. H. Adamson, R. K. Prud'homme, R. Car, D. A. Saville and I. A. Aksay, J. Phys. Chem. B, 2006, 110, 8535–8539 CrossRef CAS PubMed.
- M. F. El-Kady, V. Strong, S. Dubin and R. B. Kaner, Science, 2012, 335, 1326–1330 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details, Movie S1. See DOI: 10.1039/c3ra46027d |
| This journal is © The Royal Society of Chemistry 2014 |