The quantitative detection of HO˙ generated in a high temperature H2O2 bleaching system with a novel fluorescent probe benzenepentacarboxylic acid†
Fang Siab,
Xuan Zhang*a and
Kelu Yan*ab
aSchool of Chemistry, Chemical Engineering and biotechnology, Donghua University, Shanghai 201620, P. R. China. E-mail: xzhang@dhu.edu.cn; klyan@dhu.edu.cn; Fax: +86 021 67792619; Tel: +86 021 67792619
bNational Engineering Research Center for Dyeing and Finishing of Textiles, Donghua University, Shanghai 201620, P. R. China
First published on 19th November 2013
Abstract
A novel fluorescent probe, benzenepentacarboxylic acid (BA), was developed for the quantitative detection of hydroxyl radicals (HO˙) in a simulated hydrogen peroxide (H2O2) bleaching system. Compared to terephthalic acid (TA), a commonly-used fluorescent probe of HO˙ with four possible reaction sites, the present probe has only one reaction site for HO˙ addition that results in the formation of a single fluorescent product, hydroxybenzenepentacarboxylic acid (HBA). The generation of the single pure fluorescent product by BA, rather than mixtures by TA, made the present probe more sensitive, accurate and reproducible in the quantitative detection of HO˙. Based on the working curve obtained from the fluorescence intensity and HBA concentration, a fluorimetric method for the quantitative detection of HO˙ was developed. The present probe BA was successfully used for the quantitative detection of HO˙ under alkaline and high temperature (80 °C) conditions, in a simulated H2O2 bleaching system. The method was highly sensitive, reproducible, and applicable in a wide temperature range of 20–98 °C. The present novel fluorescent probe will be potential tool for evaluating the generation and function kinetics of HO˙ generated in biological and environmental fields.
1. Introduction
Hydroxyl radicals (HO˙) play a crucial role in the physiological and pathological processes of biological molecules such as DNA, protein and fat.1,2 Efforts on the detection of intracellular HO˙ has been made recently.3 In vitro, HO˙ has also gained great attention due to its strong oxidizing property and non-selectivity in rapid reactions with various organic and inorganic substances.4,5 However, the high reactivity and short life of HO˙6 make it difficult to realize quantitative determination directly. Most of the approaches reported for HO˙ detection in vitro are focused on the qualitative description of standard Fenton (Fe2+/H2O2) and Fenton-like systems (pH ≤ 7).7–9 Currently the developed methods for HO˙ detection mainly include electron paramagnetic resonance spectroscopy (ESR),10 chemiluminescence (CL),11 high-performance liquid chromatography (HPLC)12 and electrochemistry13 etc. However, these methods have non-ignorable defects, such as, complex operation, poor sensitivity and selectivity, and poor anti-interference ability.Recently, the fluorimetric method has been considered to be promising for HO˙ determination,14,15 because it is rapid, sensitive, selective, and easy to operate. Fluorescent dyes such as salicylfluorone16 and rhodamine 6G (ref. 17) have been used for HO˙ determination through the reduction of the fluorescence intensity, based on the strong oxidizing ability of HO˙. But these fluorescent dyes are prone to be degraded by any substance with a certain degree of oxidizing ability, causing poor selectivity and reproducibility in HO˙ detection, especially under complex conditions. Nitroxide-contained anthracene has also been utilized as a probe to detect HO˙ in the presence of dimethylsulfoxide (DMSO), where the reaction between DMSO and HO˙ can produce methyl radicals (˙CH3) that could be further captured by the fluorescent probe to enhance the fluorescence intensity.18 This indirect method needs a long duration for the multistep reaction processes, resulting in poor sensitivity and reproducibility.
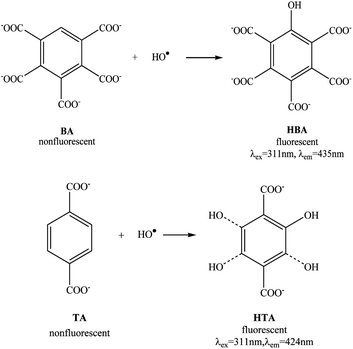
As a new class of trapping ligand, benzoic acid and terephthalic acid (TA) have been extensively employed for HO˙ detection via the formation of fluorescent hydroxylated products.19,20 There exist 5 vacant sites available for HO˙ additions on the asymmetric benzoic acid molecule, but only ortho-hydroxylated derivatives emit fluorescence. Thus, the non-selectivity in the HO˙ addition leads to the generation of non-fluorescent meta- and para-hydroxylated derivatives as well as multiple-hydroxylated derivatives. This means that invalid HO˙ addition reactions are inevitable, leading to poor sensitivity and inaccuracy in HO˙ determination. Thus symmetrical TA has been developed to replace benzoic acid as a fluorescent probe for HO˙.20 Regardless of any vacant site combined with HO˙, fluorescent ortho-hydroxylated products could be derived, making TA widely applicable in HO˙ determination in biological and environmental systems.7,20–26 However, it is obvious that multiple-hydroxylated derivatives, including double, three, and four hydroxylated derivatives should be produced simultaneously in TA systems because of the non-selectivity in the HO˙ addition. As a result, the TA-based fluorescent probe for HO˙ detection will show a poor reproducibility that limits its practical application in quantitative detection of HO˙. Hence, it is urgent to develop a novel fluorescent probe for HO˙ with excellent specificity, reproducibility and stability to realize the quantitative determination of HO˙, particularly under practical complex conditions, such as a high temperature H2O2 bleaching system which is an important pretreatment process in the textile industry to remove impurities to facilitate the subsequent dyeing and finishing processes. Unlike standard Fenton (Fe2+/H2O2) and Fenton-like reaction systems (pH ≤ 7, room temperature), the high temperature H2O2 bleaching system (20–98 °C, pH ≥ 7) is representative of practical complex conditions which have rarely been used to generate HO˙. Therefore, the development of a fluorescent probe that is capable of detecting HO˙ at a high temperature, will be helpful for monitoring the HO˙ yield under practical complex conditions, but also for understanding the H2O2 bleaching mechanism.
To get a good reproducibility from the TA system, one concept is considered that only a single pure fluorescent product is generated after addition reaction with HO˙. Bearing this in mind, we envisaged the introduction of substitution on the benzene ring of TA, leaving one site feasible for HO˙ addition, which means that a single pure fluorescent product should be obtained. Herein benzenepentacarboxylic acid (BA), which has five carboxylic acid groups on the benzene ring and only one vacant site available for HO˙ addition, was firstly used as a novel fluorescent probe for HO˙ detection. Compared with the commonly-used TA system, a superior performance such as a better water-solubility, high sensitivity, and good reproducibility, was found in the BA system, especially under high temperature conditions. The present novel probe was practically applied to the quantitative evaluation of HO˙ generation in high temperature alkaline H2O2 bleaching systems.
2. Experimental
2.1. Apparatus
The fluorescence spectra and relative fluorescence intensity was measured on an F-7000 spectrophotofluorometer (Hitachi, Japan) with a xenon lamp excitation and a quartz cuvette (1.0 cm optical path) as the container. The excitation and emission band-pass of spectrometer slits were set at 5.0 nm, with the PMT voltage of 400 V.The ESI-MS spectra were obtained on a Varian 310 mass spectrometer. The 1H NMR and 13C NMR spectra were recorded in D2O on a Bruker AVANCE III 400 MHz spectrometer with TMS as the standard. All of the H2O2 systems were heated by an RY-25012 room temperature oscillation dyeing machine (Long Ling electronic technology co., LTD, Shanghai, China).
2.2. Reagents
Benzenepentacarboxylic acid (BA, purity > 98%, TCI), terephthalic acid (TA, purity 99%), H2O2 (30 wt%), tetra-acetylethylenediamine (TAED) and dimethylsulfoxide (DMSO) were purchased from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China). Sodium benzenepentacarboxylate and terephthalate stock solution (0.01 mol l−1) were prepared from benzenepentacarboxylic acid and terephthalic acid through neutralization with NaOH, respectively. The working solution was prepared from a dilution of the corresponding stock solution. All the chemicals used were of analytical reagent grade. Double distilled water was used throughout.2.3. Procedures for HO˙ determination
H2O2 (2.2 × 10−4 mol l−1) was added into a reaction solution containing BA/TA (4 × 10−4 mol l−1) and NaOH (4 × 10−3 mol l−1). The reaction mixture was kept at a certain temperature and then promptly quenched by flowing cold water. A certain amount of reaction solution was placed in the four-pass optical quartz cuvette, then the fluorescence spectra and relative fluorescence intensity was measured.3. Results and discussion
3.1. The excitation and emission spectra
The excitation and emission spectra of BA were recorded in an alkaline solution with and without H2O2 addition. As shown in Fig. 1, BA only was nonfluorescent, whereas a strong fluorescence emission was observed in the presence of H2O2 (λex = 311 nm, λem = 435 nm), revealing that the HO˙ generated from the reaction system promoted the formation of a relative fluorescent product. | ||
| Fig. 1 The excitation and emission spectra of BA. (1) BA (4 × 10−4 mol l−1); (2) BA (4 × 10−4 mol l−1), H2O2 (2.2 × 10−4 mol l−1). T = 80 °C, pH = 10.0; t = 60 min. | ||
3.2. A comparison of the performances between the BA and TA systems
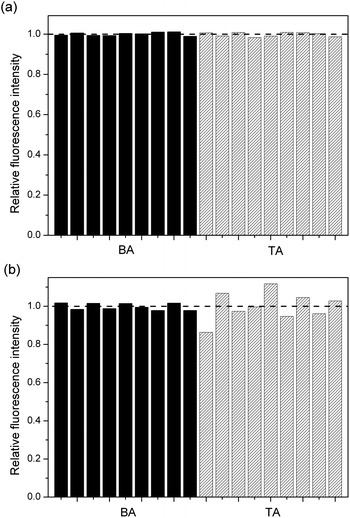
To exemplify the superior performance of BA, the results of 9 parallel determinations both in the Fenton (Fe2+/H2O2) reaction system at an ambient temperature and the alkaline H2O2 reaction system at a high temperature (80 °C), were compared by using BA and TA as the fluorescent probe to capture HO˙ respectively (Fig. 2). It can be seen that BA showed a comparable reproducibility with that of TA in the Fenton system (Fig. 2a), with a respective relative standard deviation (RSD) of 0.8% and 1.03%. However, in the high temperature alkaline H2O2 reaction system, while the reproducibility of TA became much worse (RSD, 7.59%), BA still exhibited an excellent reproducibility for HO˙ detection (RSD, 2.39%). The superiority of BA in the high temperature H2O2 reaction system may result from its one vacant site on the benzene ring allowing for HO˙ addition to generate a single fluorescent hydroxylated product HBA (Scheme 1). In contrast, various multiple-hydroxylated derivatives could be produced in the case of TA which cause a poor reproducibility, which is due to the fact that there is more than one site available for HO˙ combination (Scheme 1).To prove such an assumption, ESI-MS was used to analyse the reaction mixtures of BA and TA obtained in the high temperature system respectively (Fig. S1, ESI†). One main peak was found at m/z 470.7 for BA, which is ascribed to a single hydroxylated BA product (M + OH + 7Na)+, whereas various peaks were observed at m/z = 227.8, 242.0, 340.7 and 362.5 for TA, which are ascribed to (M + OH + 2Na)+, (M + 2OH + 2Na)+, (M + 4OH + 5Na)+ and (M + 4OH + 6Na)+. Clearly, a single hydroxylated fluorescent product was produced by BA as described in Scheme 1, whereas single and multiple-hydroxylated products were formed by TA which is different from the previous assumption that only one single hydroxylated product is formed.4,19–26 The better performance allows BA to be a novel fluorescent probe for HO˙ determination, even in severe alkaline and high temperature conditions, in a simulated H2O2 bleaching system.
DMSO has been known to be an efficient scavenger for HO˙.27 It was found that the addition of DMSO into the alkaline H2O2 reaction system efficiently quenched the fluorescence originated from hydroxylated BA (Fig. S2, ESI†), indicating the suppression effects of DMSO on the formation of fluorescent HBA. This further confirms that the fluorescence enhancement indeed results from the formation of HBA, a fluorescent product formed through the addition of HO˙ to BA molecules (Scheme 1).
3.3. Optimization of analytical parameters for HO˙ detection
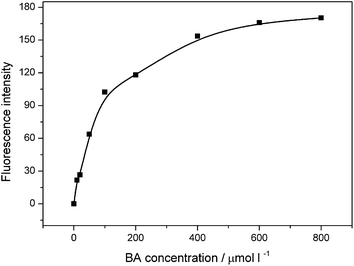
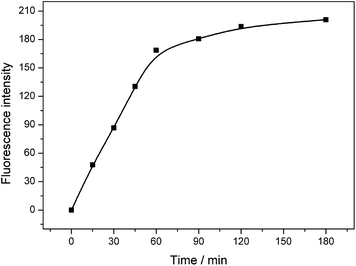
To get optimal conditions for HO˙ detection in a high temperature H2O2 bleaching system, the effect of the analytical parameters including the temperature, pH, BA concentration and reaction duration on the HO˙ yield characterized by fluorescence intensity was discussed respectively in BA systems.3.4. Quantitative determination of HO˙
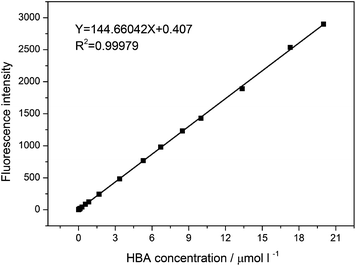
Based on the one-to-one correlation between the HO˙ and HBA amounts, the working curve for the quantitative detection of HO˙ was obtained by measuring the fluorescence intensity of HBA in a series of concentrations (4.06 × 10−3 to 20 μmol l−1) at pH 10. As displayed in Fig. 8, a good linear relationship between the HBA concentration and the fluorescence intensity was gained with R2 = 0.99979. It can be described by the equation
| Y = 144.66042X + 0.407, | (1) |
| [TAED], mg l−1 | [HO˙], μmol l−1 | ![[X with combining macron]](https://www.rsc.org/images/entities/i_char_0058_0304.gif) , μmol l−1 , μmol l−1 |
RSD, % |
|---|---|---|---|
| 0 | 1.05, 1.03, 1.01, 1.07, 1.02 | 1.04 | 2.26 |
| 10 | 1.87, 1.76, 1.86, 1.83, 1.92 | 1.85 | 3.19 |
| 20 | 2.71, 2.62, 2.55, 2.67, 2.78 | 2.66 | 3.35 |
| 30 | 4.55, 4.41, 4.35, 4.59, 4.61 | 4.5 | 2.56 |
| 40 | 5.51, 5.36, 5.46, 5.61, 5.53 | 5.49 | 1.68 |
| 60 | 6.67, 6.71, 6.57, 6.78, 6.82 | 6.71 | 1.46 |
| 80 | 7.71, 7.32, 7.43, 7.52, 7.69 | 7.53 | 2.28 |
| 100 | 8.27, 8.13, 8.1, 8.46, 8.39 | 8.27 | 1.9 |
| 200 | 8.89, 8.62, 8.92, 9.16, 8.82 | 8.88 | 2.19 |
| 300 | 9.62, 9.5, 9.37, 9.73, 9.68 | 9.58 | 1.52 |
| 400 | 9.83, 9.6, 9.89, 9.62, 9.94 | 9.78 | 1.6 |
4. Conclusions
A quantitative detection for HO˙ was proposed based on a novel fluorescent probe BA in this work. The present probe BA was for the first time used to quantitatively determine HO˙ in a high temperature alkaline H2O2 bleaching system. It proves that BA, with only one reaction site, can overcome the defects of a poor reproducibility and a lack of quantitative detection which are encountered by a previous fluorescent probe TA. The pure hydroxylated product HBA was obtained to establish the standard curve between the HO˙ concentration and the fluorescence intensity. A fluorimetric method for the quantitative detection of HO˙ was developed with an excellent reproducibility under severe conditions. It is simple, rapid, sensitive, and available to precisely detect HO˙ in a wide temperature range 20–98 °C. The present fluorescent probe will be helpful for monitoring the HO˙ yield under practical complex conditions, but also for understanding the H2O2 bleaching mechanism.Acknowledgements
The work was a part of the national science and technology support program (no. 2009BAE88B02) and is supported by the National Ministry of Science and Technology. This work is also supported by the Graduate Student Degree Thesis Innovation Foundation Projects of Donghua University (Grant no. CUSF-DH-D2013050).References
- M. Dizdaroglu and P. Jaruga, Free Radical Res., 2012, 46, 382 CrossRef CAS PubMed.
- J. Du and J. M. Gebicki, Int. J. Biochem. Cell Biol., 2004, 36, 2334 CrossRef CAS PubMed.
- L. Yuan, W. Y. Lin and J. Z. Song, Chem. Commun., 2010, 46, 7930 RSC.
- F. Ay, E. C. Catalkaya and F. Kargi, J. Hazard. Mater., 2009, 162, 230 CrossRef CAS PubMed.
- N. H. Zhang, Z. T. Zhang, M. D. Bai, C. Chen, X. Y. Meng and Y. P. Tian, Mar. Pollut. Bull., 2012, 64, 2742 CrossRef CAS PubMed.
- F. C. Cheng, J. F. Jen and T. H. Tsai, J. Chromatogr., B: Anal. Technol. Biomed. Life Sci., 2002, 781, 481 CrossRef CAS.
- B. Tang, L. Zhang and Y. Gen, Talanta, 2005, 65, 769 CrossRef CAS PubMed.
- L. X. Li, Y. Abe, Y. Nagasawa, R. Kudo, N. Usui, K. Imai, T. Mashino, M. Mochizuki and N. Miyata, Biomed. Chromatogr., 2004, 17, 470 Search PubMed.
- L. X. Li, Y. Abe, K. Kanagawa, T. Shoji, T. Mashino, M. Mochizuki, M. Tanaka and N. Miyata, Anal. Chim. Acta, 2007, 599, 315 CrossRef CAS PubMed.
- K. Q. Zhou, J. J. Yin and L. L. Yu, Food Chem., 2006, 95, 446 CrossRef CAS PubMed.
- C. J. Miller, A. L. Rose and T. D. Waite, Anal. Chem., 2011, 83, 261 CrossRef CAS PubMed.
- C. Tai, J. F. Peng, J. F. Liu, G. B. Jiang and H. Zou, Anal. Chim. Acta, 2004, 527, 73 CrossRef CAS PubMed.
- E. Kilinc, Talanta, 2005, 65, 876 CrossRef CAS PubMed.
- N. Soh, Anal. Bioanal. Chem., 2006, 386, 532 CrossRef CAS PubMed.
- X. Q. Chen, X. Z. Tian, I. Shin and J. Yoon, Chem. Soc. Rev., 2011, 40, 4783 RSC.
- F. L. Ren, N. Wu and S. H. Si, Chin. J. Anal. Chem., 2001, 29, 60 CAS.
- J. Fan, H. Q. Guo and S. L. Feng, J. Fluoresc., 2007, 17, 257 CrossRef CAS PubMed.
- X. F. Yang and X. Q. Guo, Analyst, 2001, 126, 1800 RSC.
- E. Vidrio, C. H. Phuah, A. M. Dillner and C. Anastasio, Environ. Sci. Technol., 2009, 43, 922 CrossRef CAS.
- M. Saran and K. H. Summer, Free Radical Res., 1999, 31, 429 CrossRef CAS.
- X. H. Qu, L. J. Kirschenbaum and E. T. Borish, Photochem. Photobiol., 2000, 71, 307 CrossRef CAS.
- K. R. Millington and L. J. Kirschenbaum, Rev. Prog. Color. Relat. Top., 2002, 118, 6 CrossRef CAS.
- S. E. Page, W. A. Arnold and K. McNeill, J. Environ. Monit., 2010, 12, 1658 RSC.
- S. A. V. Eremia, D. C. Lucia, G. L. Radu and J. L. Marty, Talanta, 2008, 77, 858 CrossRef CAS PubMed.
- W. J. Jiang, J. A. Joens, D. D. Dionysiou and K. E. O'Shea, J. Photochem. Photobiol., A, 2013, 262, 7 CrossRef CAS PubMed.
- T. Hirakawa, K. Yawata and Y. Nosaka, Appl. Catal., A, 2007, 325, 105 CrossRef CAS PubMed.
- L. Ren, H. K. Kim and W. W. Zhong, Anal. Chem., 2009, 81, 5510 CrossRef CAS PubMed.
- J. Z. Shao, Y. Huang, Z. H. Wang and J. Q. Liu, Color. Technol., 2010, 126, 103 CAS.
- J. Y. Cai, D. J. Evans and S. M. Smith, AATCC Rev., 2001, 1, 31 CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c3ra45975f |
| This journal is © The Royal Society of Chemistry 2014 |