A rhodamine B-based “turn-on” fluorescent sensor for detecting Cu2+ and sulfur anions in aqueous media†
Dan Guoab,
Zhengping Donga,
Chao Luoa,
Wenyan Zana,
Shiqiang Yan*a and
Xiaojun Yaoa
aCollege of Chemistry and Chemical Engineering, Lanzhou University, Lanzhou 730000, PR China. E-mail: yansq@lzu.edu.cn; Fax: +86 931 8912582; Tel: +86 931 8912582
bHuayin Ordnance Test Center, Huayin 714200, China
First published on 17th December 2013
Abstract
A novel selective fluorescent chemosensor L based on rhodamine B derivative has been designed and synthesized to optically detect Cu2+ and S2− in aqueous buffer solution. The fluorescence of L was excited by Cu2+ with a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding ratio, and could be used as a “turn-on” type sensor. Otherwise, the emission band would undergo a slight red-shift. Once binding with Cu2+, the copper complex can exhibit high selectivity for S2−. Upon addition of Cu2+, the color of the chemosensor L changes from colorless to pink. Additionally, the resulting pink solution changes to colorless immediately upon the addition of S2−. However, no phenomenon change could be observed in the presence of other anions. Conclusively, the color changes suggest that sensor L could serve as a “naked-eye” sensor for Cu2+ and S2−.
1 binding ratio, and could be used as a “turn-on” type sensor. Otherwise, the emission band would undergo a slight red-shift. Once binding with Cu2+, the copper complex can exhibit high selectivity for S2−. Upon addition of Cu2+, the color of the chemosensor L changes from colorless to pink. Additionally, the resulting pink solution changes to colorless immediately upon the addition of S2−. However, no phenomenon change could be observed in the presence of other anions. Conclusively, the color changes suggest that sensor L could serve as a “naked-eye” sensor for Cu2+ and S2−.
Introduction
Transition-metal ions are toxic, causing serious environmental and health issues.1,2 As one of most common transition-metal ions, Cu2+ is one of the highly toxic and global widespread pollutants. As the third most abundant transition metal in the human body, Cu2+ plays a vital role in many biological processes.3–5 Some reports showed that excessive levels of Cu2+ accumulated could induce severe neurodegenerative diseases, such as Menkes and Wilson's diseases, familial amyotrophic lateral sclerosis, prion disease and Alzheimer's disease.6–11 Hence, there remains a significant challenge for development of rapid detection and removal of copper from pollutants. Currently, many techniques have been reported for detecting of Cu2+ ion, including atomic absorption spectrometry, inductively coupled plasma atomic emission spectroscopy.12–16 On the other hand, fluorescent chemosensors have attracted considerable attention due to its simplicity, low-cost and sensitivity. In past few years, many chemosensors with “turn-off” signal upon binding of Cu2+ have been reported.17–19 However, it is not as sensitive as fluorescene enhancement response.20–26 Therefore, it is also necessary to design and synthesize fluorescent probes of “turn-on” type in aqueous systems, especially for the response enhancement.Recently, the hydrogen sulfide (H2S) has proved as a novel gasotransmitter, and exhibited important physiological function like nitric oxide (NO), carbon monoxide (CO) in animal systems.27,28 The sulfur anion is also an important anion in biological and environmental samples. Most environmental sulfides are released by industries which are involved in conversion of sulfur, such as preparation of sulfuric acid and dyes, cosmetic manufacturing, and production of wood pulp. On the other hand, sulfide anions can also be generated from microbial reduction of sulfate by anaerobic bacteria or from the sulfur-containing amino acids in meat protein.29 The protonated forms of sulfur, such as HS− or H2S are more toxic than sulfide. If mammal drinks the water contaminated with sulfide, it will damage mucous membranes and cause respiratory problem. For example, low concentration of hydrogen sulfide has been proven to produce dizziness, while higher concentration can cause loss of consciousness, permanent damage of brain tissues, or even suffocation.30,31 However, current major methods for H2S detection, such as inductively coupled plasma atomic emission spectroscopy and electrochemical determination, titration and ion chromatography, often require complicated sample processing, which would lead to predicament for accurate analysis of this important molecule.32–36 Therefore, development of a quick and sensitive method for immediate sulfide detection in aqueous media is of high interest.
Recently, rhodamine B-based dyes have been widely used as fluorescence reporting groups due to their excellent spectroscopic properties, such as large molar extinction coefficient, high fluorescence quantum yields, long absorption and emission wavelength.37 The sensing mechanisms of these chemosensors probes are mainly based on the structure changes of spirocyclic towards its open-cycle forms.38–45 On the grounds of these strategies, we designed a novel rhodamine B moiety of L as a “turn-on” fluorescent probe for Cu2+ sensing in aqueous solution (Scheme 1). In the absent of Cu2+ ions, the L exists in spirocyclic form, which is colorless with weak fluorescent intensity. Upon addition of Cu2+ ion into L, the spirocycle structure opened and the solution showed clear pink color, which also generated a fluorescence enhancement response. Additionally, high selectivity toward Cu2+ ion over other metal ions was clearly observed. Meanwhile, we also found L–Cu2+ complex can be employed to detect sulfide. By adding sulfide to the solution of L–Cu2+ complex, the color of the aqueous change from pink to colorless.
Experimental
Reagents
All reagents and solvents were purchased from commercial suppliers. All chemicals used in this work were of analytical reagent grade and used without further purification. The solutions of the metal ions were prepared from NaNO3, Mg(NO3)2·6H2O, Al(NO3)3·9H2O, KNO3, Ca(NO3)2·4H2O, Cr(NO3)3·9H2O, MnCl2·4H2O, Fe(NO3)3·9H2O, FeCl2·4H2O, Co(NO3)2·6H2O, Ni(NO3)2·6H2O, Cu(NO3)2·3H2O, Zn(NO3)2·6H2O, AgNO3, Cd(NO3)2·4H2O, Ba(NO3)2, Hg(NO3)2·0.5H2O, Pb(NO3)2. The solutions of anions were prepared from NaNO2, NaNO3, KCN, KSCN, Na2SO4, Na2SO3, Na3PO4·12H2O, Na2HPO4, KH2PO4, NaAc, NaCl, NaBr, KI, Na2S. Tris–HCl buffer solutions (1 × 10−2 mol L−1, pH 7.2) and all other solutions were prepared in deionized water.Instruments
1H and 13C NMR spectra were taken on a Varian Mercury 300 MHz NMR spectrometer in CDCl3 solutions, with tetramethylsilane (TMS, 0.00 ppm) as an internal standard. Absorption spectra were determined on a Varian UV-Cary100 UV-vis spectrophotometer. Fluorescence spectra measurements were performed on a Hitachi F-4500 fluorescence spectrophotometer. Mass spectra were obtained on a Bruker Daltonics-esquire 6000 mass spectrometer. All pH values were recorded on a PHS-3C digital pH meter (Shanghai, China). All spectra were recorded at room temperature.Synthesis of compound 2
Compound 2 was synthesized based on the reported procedures with minor changes.46 The process is the following steps: p-cresol (33 g, 0.305 mol) were completely dissolved in sodium hydroxide solution (10 mol L−1, 50 ml). Following full development of a gold color, paraformaldehyde (18 g, 0.6 mol) was added to the solution. The resulting solution was heated to 323 K for 1 hour with a magnetic stirrer bar, and then cooled to room temperature. The yellow solid was collected and dissolved again in hot water. The pH value was adjusted to 3 with HCl (2 mol L−1). The yellow precipitate was collected by filtration and dried overnight in a vacuum oven at 323 K, which afforded a creamy yellow solid compound 1. Then, compound 1 (10 g, 0.06 mol) was added to 300 ml of CHCl3 in a 500 ml round bottom flask, afterwards, activated MnO2 (21.7 g, 0.25 mol) was added to the solution with mechanically stirring and refluxing overnight, after which the reaction was cooled to room temperature and filtered, washed with CHCl3 thoroughly until the filtrate became colorless. After the solvent was evaporated under reduced pressure, the crude product was purified by flash column chromatography on silica gel (petroleum ether–ethyl acetate = 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v), affording compound 2 as yellow solid (6.27 g, yields: 62.89%). 1HNMR (CDCl3, 400 MHz): δ 2.22 (s, 3H, Ar–CH3), 3. 01 (s, H, CH2–OH), 4.60 (s, 2H, Ar–CH2), 7.15, (s, 1H, Ar–H), 7.30 (s, 1H, Ar–H), 9.72 (s, 1H, N
1, v/v), affording compound 2 as yellow solid (6.27 g, yields: 62.89%). 1HNMR (CDCl3, 400 MHz): δ 2.22 (s, 3H, Ar–CH3), 3. 01 (s, H, CH2–OH), 4.60 (s, 2H, Ar–CH2), 7.15, (s, 1H, Ar–H), 7.30 (s, 1H, Ar–H), 9.72 (s, 1H, N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–H), 11.03 (s, 1H, Ar–OH) ppm. 13C NMR (CDCl3, 100 MHz): δ 20.09, 59.96, 119.86, 128.88, 128.97, 132.38, 136.66, 157.00, 196.59 ppm.
C–H), 11.03 (s, 1H, Ar–OH) ppm. 13C NMR (CDCl3, 100 MHz): δ 20.09, 59.96, 119.86, 128.88, 128.97, 132.38, 136.66, 157.00, 196.59 ppm.
Synthesis of rhodamine sensor (compound L)
RBH was synthesized from rhodamine B according to the literature method.47RBH (0.30 g, 0.66 mmol) was dissolved in 15 ml absolute ethanol. Excess compound 2 (0.16 g, 0.99 mmol) was added to the reaction mixture, then the mixture was stirred and reflux under N2 atmosphere for 6 hour. Then the solvent was removed under reduced pressure, and the residue was purified by recrystallization with ethanol as yellow solid (0.29 g, yields: 72.7%). 1HNMR (400 MHz, CDCl3): δ 1.19–1.13 (t, J = 7.0 Hz, 12H, NCH2CH3), 2.19 (s, 3H, ben–CH3), 2.75 (s, 1H, ben–CH2–OH), 3.37–3.29 (q, J = 7.0 Hz, 8H, NCH2CH3), 4.62–4.64 (d, J = 5.5 Hz, 2H, ben–CH2–OH), 6.25–6.52 (m, 6H, xanthene–H), 6.82–6.79 (d, J = 1.1 Hz, 1H, ben–H), 6.98 (s, 1H, ben–H), 7.11–7.19 (m, 1H, Ar–H), 7.47–7.54 (td, J1 = 6.5, J2 = 1.2 Hz, 2H, Ar–H), 7.93–8.04 (m, 1H, Ar–H), 8.91 (s, 1H, N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–H), 11.17 (s, 1H, ben–OH); 13C NMR (101 MHz, CDCl3): δ 12.60, 20.23, 44.38, 62.09, 66.16, 97.99, 105.13, 108.27, 118.00, 123.37, 124.04, 127.86, 128.09, 128.23, 128.53, 129.37, 130.81, 131.24, 133.56, 149.10, 151.30, 151.83, 153.32, 154.46, 164.33. ESI-MS spectrometry showed a peak with m/z 605.6 [M + H]+, calculate for C37H40N4O4 = 604.74. Anal. calcd for C37H40N4O4 (604.74): C 73.42, H 6.61, N 9.26, O 10.71 found: C 73.21, H 6.65, N 9.06, O 11.08%.
C–H), 11.17 (s, 1H, ben–OH); 13C NMR (101 MHz, CDCl3): δ 12.60, 20.23, 44.38, 62.09, 66.16, 97.99, 105.13, 108.27, 118.00, 123.37, 124.04, 127.86, 128.09, 128.23, 128.53, 129.37, 130.81, 131.24, 133.56, 149.10, 151.30, 151.83, 153.32, 154.46, 164.33. ESI-MS spectrometry showed a peak with m/z 605.6 [M + H]+, calculate for C37H40N4O4 = 604.74. Anal. calcd for C37H40N4O4 (604.74): C 73.42, H 6.61, N 9.26, O 10.71 found: C 73.21, H 6.65, N 9.06, O 11.08%.
Results and discussion
UV-vis spectroscopic studies of L in presence of Cu2+
The spectra responses of chemosensor L in the absence or presence of Cu2+ in different pH values were evaluated first. As shown in Fig. 1, the absorption of free L was very weak between pH 4 and 10. When the pH value was lower than 4, it showed increased absorption. It was attributed to the ring opening of rhodamine occurred at acid conditions (pH < 4) for strong protonation (Scheme S1†). However, the absorbance intensity had significant enhancement after the addition of Cu2+ ion between pH 4 and 9. It was due to the formation of ring-opened L–Cu2+ complex (Scheme 2). Then under basic conditions, the absorbance intensity decreased due to the formation of Cu(OH)2. The data indicated that L has good absorption response for Cu2+ under physiological pH conditions. Therefore, further UV-vis studies were carried out in CH3CN/Tris–HCl solution (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v, pH = 7.2).
1 v/v, pH = 7.2).
 | ||
Fig. 1 UV-vis absorption of free L (2 × 10−5 mol L−1) and L + 5 equiv. of Cu2+ ion in CH3CN/Tris–HCl solution (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v) with different pH conditions. The absorption wavelength is 556 nm. 1 v/v) with different pH conditions. The absorption wavelength is 556 nm. | ||
The solution of L in CH3CN/Tris–HCl solution (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
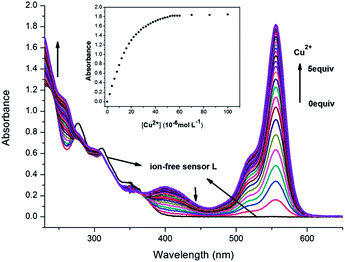
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v, pH = 7.2) is colorless. As shown in Fig. 2, the absorption spectra of L alone (2 × 10−5 mol L−1) exhibited no band in the region beyond 500 nm, which indicated that L was of the spirolactam form. The addition of Cu2+ into the solution immediately resulted in a strong absorption band centered at 556 nm, with an obvious color change from colorless to pink. A slight increase at 555 nm after the addition of Fe2+ was also observed at the same concentration. We infer that Fe2+ ion have lower binging affinity to L compared with Cu2+ in aqueous media. Under the same conditions, no obvious response could be observed upon the addition of other ions. Therefore, L can serve as a “naked-eye” indicator for Cu2+ ion. Even in the presence of miscellaneous competitive metal ions, the addition of Cu2+ ion still resulted in a large absorption change at 556 nm (Fig. 3). This indicates that the selectivity of L to Cu2+ ion is excellent in the presence of other competitive cations in acetonitrile–water medium.
1 v/v, pH = 7.2) is colorless. As shown in Fig. 2, the absorption spectra of L alone (2 × 10−5 mol L−1) exhibited no band in the region beyond 500 nm, which indicated that L was of the spirolactam form. The addition of Cu2+ into the solution immediately resulted in a strong absorption band centered at 556 nm, with an obvious color change from colorless to pink. A slight increase at 555 nm after the addition of Fe2+ was also observed at the same concentration. We infer that Fe2+ ion have lower binging affinity to L compared with Cu2+ in aqueous media. Under the same conditions, no obvious response could be observed upon the addition of other ions. Therefore, L can serve as a “naked-eye” indicator for Cu2+ ion. Even in the presence of miscellaneous competitive metal ions, the addition of Cu2+ ion still resulted in a large absorption change at 556 nm (Fig. 3). This indicates that the selectivity of L to Cu2+ ion is excellent in the presence of other competitive cations in acetonitrile–water medium.
Titration experiment showed that the absorption spectra of L to Cu2+ ion also gradually increased at 401 nm and 556 nm, with higher Cu2+ concentration (0–10 × 10−5 mol L−1) (Fig. 4). The absorbance at 556 nm had an approximate 643-fold enhancement. The Job's plot was conducted to determine the binding stoichiometry of the L–Cu2+ complex, wherein the total concentration of L and Cu2+ ion is 4 × 10−5 mol L−1 and the mole fraction of Cu2+ ion is in the range from 0 to 1. As shown in Fig. 5, we can observe that the absorbance went through a maximum peak at a molar fraction of about 0.5, indicating a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding stoichiometry between Cu2+ and L. To achieve this stoichiometry, carbonyl O, imino N, and phenol O atoms of L are the most likely binding sites for Cu2+. The proposed structure of L–Cu2+ is illustrated in Scheme 2.
1 binding stoichiometry between Cu2+ and L. To achieve this stoichiometry, carbonyl O, imino N, and phenol O atoms of L are the most likely binding sites for Cu2+. The proposed structure of L–Cu2+ is illustrated in Scheme 2.
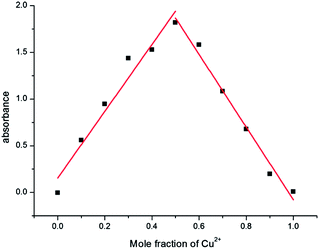 | ||
Fig. 5 Job's plot of L and Cu2+ in CH3CN/Tris–HCl solution (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v, pH = 7.2). The total concentration of L and Cu2+ ions is 4 × 10−5 mol L−1. The absorbance was collected at 556 nm. 1 v/v, pH = 7.2). The total concentration of L and Cu2+ ions is 4 × 10−5 mol L−1. The absorbance was collected at 556 nm. | ||
Assuming a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 association between L and Cu2+, the association constant (Ka) of L–Cu2+ was determined using the Benesi–Hildebrand equation as follows.48
1 association between L and Cu2+, the association constant (Ka) of L–Cu2+ was determined using the Benesi–Hildebrand equation as follows.48
 | (1) |
A and A0 represent the absorbance of L solution in the presence and absence of Cu2+ ion, and Amax is the saturated absorbance of L in the presence of excess amount of Cu2+. [Cu2+] is the concentration of Cu2+ ion added. Plotting of 1/(A − A0) versus 1/[Cu2+] showed a linear relationship (Fig. 6), which indicates that L bound with Cu2+ in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding stoichiometry, and the association constant Ka is determined from the slope as 6.47 × 104 M−1. The ESI-mass spectra of L–Cu2+ also showed a 1
1 binding stoichiometry, and the association constant Ka is determined from the slope as 6.47 × 104 M−1. The ESI-mass spectra of L–Cu2+ also showed a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometry. The unique peak at m/z = 666.18, (calcd = 666.27) corresponding to [CuL]+ was clearly observed when Cu2+ was added to L (Fig. S6†).
1 stoichiometry. The unique peak at m/z = 666.18, (calcd = 666.27) corresponding to [CuL]+ was clearly observed when Cu2+ was added to L (Fig. S6†).
 | ||
Fig. 6 Benesi–Hildebrand plot (absorbance at 556 nm) of L using eqn (1), assuming 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometry for association between L and Cu2+. 1 stoichiometry for association between L and Cu2+. | ||
Fluorescence spectroscopic studies of L in presence of Cu2+
The effects of pH on the sensor L was evaluated first. Fig. 7 shows that for ion-free sensor L, the fluorescence intensity was strong at pH < 6. It was due to the ring opening of rhodamine for the strong protonation. The fluorescence intensities of ion-free sensor L gradually decreased with the increase of pH value.49 In the pH range from 6 to 10, the fluorescence signal of L without Cu2+ ion was weak. Upon the addition of Cu2+ ion, there was an obvious fluorescence emission at 581 nm in pH from 6 to 9. This result suggested that L could act as a fluorescent probe for Cu2+ under physiological conditions (i.e. pH 7.2). The experiments have also been conducted in Tris–HCl solutions, wherein the selectivity of sensor L towards Cu2+ is also good. However, the fluorescence intensity in Tris–HCl solutions is not as strong as that in mixed solvents of acetonitrile and Tris–HCl buffer. Therefore, further fluorescent studies were also carried out in CH3CN/Tris–HCl solution (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v, pH = 7.2).
1 v/v, pH = 7.2).
L in CH3CN/Tris–HCl solution (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
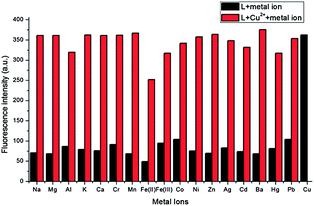
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v, pH = 7.2) showed a very weak fluorescence in the absence of metal ions when excited at 530 nm. However, the addition of Cu2+ ion resulted in remarkably enhanced fluorescence intensity. Under the same condition, additions of other metal ions including Na+, Mg2+, Al3+, K+, Ca2+, Cr3+, Mn2+, Fe3+, Co2+, Ni2+, Zn2+, Ag+, Cd2+, Ba2+, Hg2+ and Pb2+ did not cause any discernible changes. On the other hand, the Fe2+–ligand complex, in contrast to the rest of the ions in study, exhibited lower fluorescence intensity than the free ligand (Fig. 8). Similarly, parallel experiments in the presence of potentially competitive metal ions were also carried out (Fig. 9) and the results suggested that the fluorescent recognition of Cu2+ by L was hardly influenced by other co-existing metal ions.
1 v/v, pH = 7.2) showed a very weak fluorescence in the absence of metal ions when excited at 530 nm. However, the addition of Cu2+ ion resulted in remarkably enhanced fluorescence intensity. Under the same condition, additions of other metal ions including Na+, Mg2+, Al3+, K+, Ca2+, Cr3+, Mn2+, Fe3+, Co2+, Ni2+, Zn2+, Ag+, Cd2+, Ba2+, Hg2+ and Pb2+ did not cause any discernible changes. On the other hand, the Fe2+–ligand complex, in contrast to the rest of the ions in study, exhibited lower fluorescence intensity than the free ligand (Fig. 8). Similarly, parallel experiments in the presence of potentially competitive metal ions were also carried out (Fig. 9) and the results suggested that the fluorescent recognition of Cu2+ by L was hardly influenced by other co-existing metal ions.
 | ||
Fig. 8 Fluorescence of L (2 × 10−5 mol L−1) in the presence of different metal ions (1 × 10−4 mol L−1) in CH3CN/Tris–HCl solution (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v, pH = 7.2). The excitation wavelength is 530 nm. 1 v/v, pH = 7.2). The excitation wavelength is 530 nm. | ||
From the fluorescence titration experiments (Fig. 10), upon addition of Cu2+ into CH3CN/Tris–HCl solution (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v, pH = 7.2) of L, a new emission band centered at 572 nm (the excitation wavelength is 530 nm) was developed. Similar to the absorption response, the fluorescence intensity increased gradually with higher Cu2+ concentration. Meanwhile, the emission band underwent a slightly red-shifted from 572 to 581 nm after 2 equiv. of Cu2+ were added. The fluorescence intensity at 581 nm had an approximate 7-fold enhancement. The red-shift of the emission peak can be ascribed to the recombination of the orbitals after the formation of ring-opened L–Cu2+ complex. The emission intensity of ion-free sensor L was measured 10 times and the standard deviation of blank measurements was determined. The fluorescence intensity of L (2 × 10−5 mol L−1) at 581 nm was found to increase linearly with the concentration of Cu2+ in range of 0–0.8 × 10−5 mol L−1 (R2 = 0.9910) (Fig. S7†). The detection limit was then calculated with the eqn (2):31
1 v/v, pH = 7.2) of L, a new emission band centered at 572 nm (the excitation wavelength is 530 nm) was developed. Similar to the absorption response, the fluorescence intensity increased gradually with higher Cu2+ concentration. Meanwhile, the emission band underwent a slightly red-shifted from 572 to 581 nm after 2 equiv. of Cu2+ were added. The fluorescence intensity at 581 nm had an approximate 7-fold enhancement. The red-shift of the emission peak can be ascribed to the recombination of the orbitals after the formation of ring-opened L–Cu2+ complex. The emission intensity of ion-free sensor L was measured 10 times and the standard deviation of blank measurements was determined. The fluorescence intensity of L (2 × 10−5 mol L−1) at 581 nm was found to increase linearly with the concentration of Cu2+ in range of 0–0.8 × 10−5 mol L−1 (R2 = 0.9910) (Fig. S7†). The detection limit was then calculated with the eqn (2):31
| Detection limit = 3σ/k | (2) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v, pH = 7.2) was measured to be 2.43 × 10−8 mol L−1.
1 v/v, pH = 7.2) was measured to be 2.43 × 10−8 mol L−1.
Theoretical calculations
In order to further verify the configuration of L–Cu2+, we carried out density functional theory (DFT) calculations with the B3LYP exchange functions using Gaussian 09 package, and introduce the LANL2DZ effective core potential (ECP) to represent the core electrons of the Cu atom. The valence electrons are described by LANL2DZ basis set, while all the other atoms are described commonly by 6-31G(d) basis set. All the thermodynamic data are obtained at this computational level. The molecule forms a planar structure, as displayed in Fig. 11. The Cu–N bond length is 1.975 Å, and the distances of Cu–O1, Cu–O2, Cu–O3 are 1.992, 1.912, 1.978 Å, respectively. The optimized configuration shows that Cu2+ ions occupy the acylhydrazone coordination centers of L at the same time.UV-vis spectroscopic studies of L–Cu2+ complex in presence of S2−
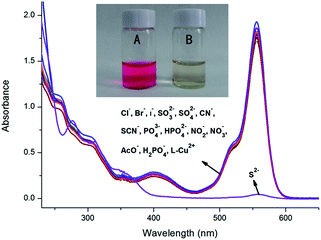
We have further studied the influence of different anions on UV-vis absorbance of L–Cu2+ complex. Upon addition of Na2S to L (2 × 10−5 mol L−1) and Cu2+ (4 × 10−5 mol L−1) complex in CH3CN/Tris–HCl solution (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v, pH = 7.2), the UV-vis absorption was decreased and the color of the L–Cu2+ complex changed from pink to colorless. The optical properties of the L–Cu2+ complex were also studied in the presence of different anions such as CN−, SCN−, SO32−, SO42−, PO43−, HPO42−, H2PO4−, Cl−, Br−, I−, NO2−, NO3−, and AcO−. It is worth noted that only by adding S2− into the solution of L–Cu2+ could cause this change, whereas other anions failed to produce any discernible spectral change (Fig. 12). For further investigation, a solution of L in CH3CN/Tris–HCl buffer (1
1 v/v, pH = 7.2), the UV-vis absorption was decreased and the color of the L–Cu2+ complex changed from pink to colorless. The optical properties of the L–Cu2+ complex were also studied in the presence of different anions such as CN−, SCN−, SO32−, SO42−, PO43−, HPO42−, H2PO4−, Cl−, Br−, I−, NO2−, NO3−, and AcO−. It is worth noted that only by adding S2− into the solution of L–Cu2+ could cause this change, whereas other anions failed to produce any discernible spectral change (Fig. 12). For further investigation, a solution of L in CH3CN/Tris–HCl buffer (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
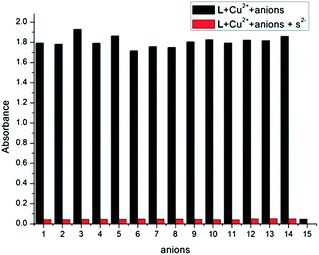
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v, pH = 7.2) containing 2 equiv. of Cu2+ was titrated by the solution of Na2S. The UV-vis spectral pattern of the titration experiment was similar but in reverse direction to the titration curve obtained with Cu2+ (Fig. 13). Upon the addition of S2− to L–Cu2+ complex, black precipitate was formed. This phenomenon showed that Cu2+ released from complex L–Cu2+ was captured by sulfide anion to produce CuS. The formation of CuS was also ascertained by the XRD measurement. The sensing capability of L–Cu2+ complex was further tested in the presence of other anions, which may interfere the estimation of copper and sulfide (Fig. 14). The receptor L–Cu2+ complex is well selective in detecting sulfur in the presence of other competitive anions. The mass spectrum of the L–Cu2+ system was also studied in the presence of S2−. ESI-MS of the above media displayed a molecular peak [L + H+] at m/z 605.6 and a molecular-ion peak [L + Na+] at m/z 627.5 which confirmed the identity of free L (Fig. S8†).
1 v/v, pH = 7.2) containing 2 equiv. of Cu2+ was titrated by the solution of Na2S. The UV-vis spectral pattern of the titration experiment was similar but in reverse direction to the titration curve obtained with Cu2+ (Fig. 13). Upon the addition of S2− to L–Cu2+ complex, black precipitate was formed. This phenomenon showed that Cu2+ released from complex L–Cu2+ was captured by sulfide anion to produce CuS. The formation of CuS was also ascertained by the XRD measurement. The sensing capability of L–Cu2+ complex was further tested in the presence of other anions, which may interfere the estimation of copper and sulfide (Fig. 14). The receptor L–Cu2+ complex is well selective in detecting sulfur in the presence of other competitive anions. The mass spectrum of the L–Cu2+ system was also studied in the presence of S2−. ESI-MS of the above media displayed a molecular peak [L + H+] at m/z 605.6 and a molecular-ion peak [L + Na+] at m/z 627.5 which confirmed the identity of free L (Fig. S8†).
Conclusions
In summary, a rhodamine-based fluorimetric probe L was designed and synthesized. Studies showed that L exhibited highly selective binding with Cu2+ over other metal ions with a fluorescence turn-on effect. The chemosensor L displayed a one-to-one complex formation with Cu2+ ions in a broad pH range. Obvious increases in colorimetric changes were observed upon the addition of Cu2+ into the CH3CN/Tris–HCl solution (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v, pH = 7.2) of chemosensor L. The complex formed between L and Cu2+ is dissociable only in the presence of sulfide anion and the color changed from pink to colorless, which makes the L–Cu2+ complex an efficient sensor for sulfide anions.
1 v/v, pH = 7.2) of chemosensor L. The complex formed between L and Cu2+ is dissociable only in the presence of sulfide anion and the color changed from pink to colorless, which makes the L–Cu2+ complex an efficient sensor for sulfide anions.
Acknowledgements
The authors acknowledge financial support from the NSFC (Grants 21301082), the Natural Science Foundation of Gansu (no. 1308RJYA028) and Huayin Ordnance Test Center. We thank Prof. Renqi Wang for giving many suggestions on this work.Notes and references
- Q. Zhao, F. Y. Li and C. H. Huang, Chem. Soc. Rev., 2010, 39, 3007–3030 RSC.
- D. T. Quang and J. S. Kim, Chem. Rev., 2010, 110, 6280–6301 CrossRef CAS PubMed.
- S. J. Lippard and J. M. Berg, Principles of Bioinorganic Chemistry, University Science Books, Mill Valley, CA, 1994 Search PubMed.
- E. L. Que, D. W. Domaille and C. J. Chang, Chem. Rev., 2008, 108, 1517–1549 CrossRef CAS PubMed.
- M. C. Linder and M. Hazegh-Azam, Am. J. Clin. Nutr., 1996, 63, 797S–811S CAS.
- E. Gaggelli, H. Kozlowski, D. Valensin and G. Valensin, Chem. Rev., 2006, 106, 1995–2044 CrossRef CAS PubMed.
- D. R. Brown and H. Kozlowski, Dalton Trans., 2004, 1907–1917 RSC.
- G. L. Millhauser, Copper binding in the prion protein, Acc. Chem. Res., 2004, 37, 79–85 CrossRef CAS PubMed.
- R. Uauy, M. Olivares and M. Gonzalez, Am. J. Clin. Nutr., 1998, 67, 952S–959S CAS.
- K. J. Barnham, C. L. Masters and A. I. Bush, Nat. Rev. Drug Discovery, 2004, 3, 205–214 CrossRef CAS PubMed.
- B. E. Kim, T. Nevitt and D. J. Thiele, Nat. Chem. Biol., 2008, 4, 176–185 CrossRef CAS PubMed.
- J. R. Chen and K. C. Teo, Anal. Chim. Acta, 2001, 450, 215–222 CrossRef CAS.
- T. W. Lin and S. D. Huang, Anal. Chem., 2001, 73, 4319–4325 CrossRef CAS.
- O. Abollino, M. Aceto, M. C. Bruzzoniti, E. Mentasti and C. Sarzanini, Anal. Chim. Acta, 1998, 375, 299–306 CrossRef CAS.
- A. Vaisanen, R. Suontamo, J. Silvonen and J. Rintala, Anal. Bioanal. Chem., 2002, 373, 93–97 CrossRef PubMed.
- R. Krämer, Angew. Chem., Int. Ed., 1998, 37, 772–773 CrossRef.
- N. Shao, Y. Zhang, S. M. Cheung, R. H. Yang, W. H. Chan and T. Mo, et al., Anal. Chem., 2005, 77, 7294–7303 CrossRef CAS PubMed.
- S. H. Kim, J. S. Kim, S. M. Park and S. K. Chang, Org. Lett., 2006, 8, 371–374 CrossRef CAS PubMed.
- Y. Luo, Y. Li, B. Q. Lv, Z. D. Zhou, D. Xiao and M. M. F. Choi, Microchim. Acta, 2009, 164, 411–417 CrossRef CAS.
- C. W. Yu, J. Zhang, R. Wang and L. X. Chen, Org. Biomol. Chem., 2010, 8, 5277–5279 CAS.
- J. Zhang, C. W. Yu, S. Y. Qian, G. Lu and J. L. Chen, Dyes Pigm., 2012, 92, 1370–1375 CrossRef CAS PubMed.
- Z. P. Dong, X. Tian, Y. Z. Chen, J. R. Hou and J. T. Ma, RSC Adv., 2013, 3, 2227–2233 RSC.
- C. W. Yu, L. X. Chen, J. Zhang, J. H. Li, P. Liu and W. H. Wang, et al., Talanta, 2011, 85, 1627–1633 CrossRef CAS PubMed.
- Z. Q. Hu, X. M. Wang, Y. C. Feng, L. Ding and H. Y. Lu, Dyes Pigm., 2011, 88, 257–261 CrossRef CAS PubMed.
- H. Zhu, J. L. Fan, J. Lu, M. M. Hu, J. F. Cao, J. Wang, H. L. Li, X. J. Liu and X. J. Peng, Talanta, 2012, 93, 55–61 CrossRef CAS PubMed.
- G. Sivaraman, T. Anand and D. Chellappa, RSC Adv., 2013, 3, 17029–17033 RSC.
- A. Kawabataa, T. Ishikia, K. Nagasawaa, S. Yoshidab, Y. Maedaa and T. Takahashia, et al., Pain, 2007, 132, 74–81 CrossRef PubMed.
- R. Wang, Antioxidants and Redox Signaling, 2003, vol. 5, pp. 493–501 Search PubMed.
- Hydrogen Sulfide, World Health Organization, Geneva, 1981, (Environmental Health Criteria, no. 19) Search PubMed.
- P. A. Patnaik, Comprehensive Guide to the Hazardous Properties of Chemical Substances, Wiley, New York, 3rd edn, 2007 Search PubMed.
- C. Kar, M. D. Adhikari, A. Ramesh and G. Das, Inorg. Chem., 2013, 52, 743–752 CrossRef CAS PubMed.
- M. Colon, J. L. Todoli, M. Hidalgo and M. Iglesias, Anal. Chim. Acta, 2008, 609, 160–168 CrossRef CAS PubMed.
- N. S. Lawrence, R. P. Deo and J. Wang, Anal. Chim. Acta, 2004, 517, 131–137 CrossRef CAS PubMed.
- S. Balasubramanian and V. Pugalenthi, Water Res., 2000, 34, 4201–4206 CrossRef CAS.
- Z. Pawlak and A. S. Pawlak, Talanta, 1999, 48, 347–353 CrossRef CAS.
- C. Giuriati, S. Cavalli, A. Gorni, D. Badocco and P. Pastore, J. Chromatogr. A, 2004, 1023, 105–112 CrossRef CAS PubMed.
- Y. Zhao, Y. Sun, X. Lv, Y. L. Liu, M. L. Chen and W. Guo, Org. Biomol. Chem., 2010, 8, 4143–4147 CAS.
- H. Li, J. Fan, J. Du, K. Guo, S. Sun, X. Liu and X. Peng, Chem. Commun., 2010, 46, 1079–1081 RSC.
- Z. Q. Hu, X. M. Wang, Y. C. Feng, L. Ding and H. Y. Lu, Dyes Pigm., 2011, 88, 257–261 CrossRef CAS PubMed.
- W. Y. Liu, H. Y. Li, B. X. Zhao and J. Y. Miao, Org. Biomol. Chem., 2011, 9, 4802–4805 CAS.
- H. N. Kim, M. H. Lee, H. J. Kim, J. S. Kim and J. Yoon, Chem. Soc. Rev., 2008, 37, 1465–1472 RSC.
- M. Beija, C. A. M. Afonso and J. M. G. Martinho, Chem. Soc. Rev., 2009, 38, 2410–2433 RSC.
- G. Sivaraman, V. Sathiyaraja and D. Chellappa, J. Lumin., 2014, 145, 480–485 CrossRef CAS PubMed.
- G. Sivaraman, T. Anand and D. Chellappa, Analyst, 2012, 137, 5881–5884 RSC.
- G. Sivaraman and D. Chellappa, J. Mater. Chem. B, 2013, 1, 5768–5772 RSC.
- L. Ma, L. P. Lu, M. L. Zhu, Q. M. Wang and Y. Li, et al., Dalton Trans., 2011, 40, 6532–6540 RSC.
- X. F. Yang, X. Q. Guo and Y. B. Zhao, Talanta, 2002, 57, 883–890 CAS.
- H. A. Benesi and J. H. Hildebrand, J. Am. Chem. Soc., 1949, 71, 2703–2707 CrossRef CAS.
- M. Z. Tian, X. J. Peng, J. L. Fan, J. Y. Wang and S. G. Sun, Dyes Pigm., 2012, 95, 112–115 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c3ra45931d |
| This journal is © The Royal Society of Chemistry 2014 |