Titanium isopropoxide complexes supported by pyrrolyl Schiff base ligands: syntheses, structures, and antitumor activity†
Abstract
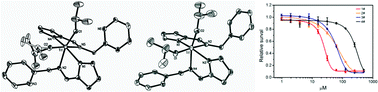
The syntheses, structures and antitumor activity of four titanium isopropoxide complexes supported by pyrrolyl Schiff base ligands are reported. Treatment of Ti(OiPr)4 with 2 equivalents of HL1 (HL1 = N-(1H-pyrrol-2-ylmethylene)(2-pyridinyl)methanimine), HL2 (HL2 = 2-pyrrolecarbaldmethylimine), HL3 (HL3 = N-((1H-pyrrol-2-yl)methylene)(phenyl)methanimine) and HL4 (HL4 = N-((1H-pyrrol-2-yl)methylene)-2-phenylethanimine), respectively, results in the formation of Ti(OiPr)2(L1)2 (1), Ti(OiPr)2(L2)2 (2), Ti(OiPr)2(L3)2 (3) and Ti(OiPr)2(L4)2 (4). All complexes have been characterized by elemental analyses and NMR studies. The solid-state structures of complexes 1 and 3 have been further established by single X-ray crystallography. The cytotoxicity activities of 1–4 towards the tumor cells HCT-116, PC3 and MCF-7 were measured. Complexes 1, 2 and 3 showed good antitumor properties.

 Please wait while we load your content...
Please wait while we load your content...