Highly efficient and chemoselective transfer hydrogenation of nitroarenes at room temperature over magnetically separable Fe–Ni bimetallic nanoparticles†
Dhananjay R. Petkar,
Brijesh S. Kadu and
Rajeev C. Chikate*
Nanoscience Group, Department of Chemistry, Post-graduate and Research Centre, MES Abasaheb Garware College, Karve Road, Pune-411004, India. E-mail: rajuchikate29@gmail.com; Fax: +91-20-25438165; Tel: +91-20-41038263
First published on 26th November 2013
Abstract
A highly chemoselective catalytic transfer hydrogenation (CTH) of nitroarenes to corresponding amino derivatives is achieved with Fe–Ni bimetallic nanoparticles (Fe–Ni NP's) as the catalyst and NaBH4 at room temperature. Their catalytic efficiency is ascribed to the presence of Ni sites on the bimetallic surface that not only hinder the surface corrosion of the iron sites but also facilitate efficient electron flow from the catalyst surface to the adsorbed nitro compounds. This facet is corroborated with reusability studies as well as surface characterization of the catalyst before and after its repetitive usage. Thus, these nanoparticles efficiently catalyze the reduction of functionalized nitroarenes to corresponding amines without use of corrosive agents like base or other additives under ambient conditions and are easily separated by a laboratory magnet in an eco-friendly manner.
1. Introduction
Selective catalytic hydrogenation of functional nitroarenes to anilines is an industrially important process for synthesis of agrochemicals, pharmaceuticals, dyestuffs, urethanes and other industrially important products.1–3 Conventionally, this reduction is carried out with Fe/HCl that requires a stoichiometric excess of reagents which eventually produces a large amount of secondary waste and therefore, is not considered an environmentally benign process. RANEY® nickel is widely used as the catalyst but has two major disadvantages: moisture sensitivity and a pyrophoric nature. Pd/C is also known to catalyze this reaction but it is more expensive and also quite sensitive to trace impurities. Besides Pd, a number of other transition metal catalysts like Pt, Ru are also reported to be efficient for this transformation. However, these methodologies require a cumbersome experimental set up like an autoclave and the process is carried out under high temperature–pressure conditions in the presence of a catalyst and H2 gas.4,5 Concomitantly, this conversion is also reported to occur at room temperature with H2 gas with a variety of catalysts6 as well as for other types of reactions such as alkenes, alkynes, olefins, ketones and cyclohexene with 90–100% conversion.7–9In order to overcome these lacunas, an alternative approach is adopted wherein various hydrogen donors like hydrazine, ammonium formate, IPA and NaBH4 are used for liquid phase catalytic transfer hydrogenation (CTH) of functional aromatics. This approach is considered to be safe, cost-effective, selective and eco-friendly. Employing this strategy, a highly chemoselective reduction of aromatic nitro compounds to the corresponding amino derivatives is achieved by a combination of copper nanoparticles and ammonium formate10 in ethylene glycol at 120 °C. On the other hand, selectivity for a Rh nanocatalyst is demonstrated for the reduction of nitroarenes with hydrazine1 monohydrate at 60 °C while a similar potentiality is exhibited by Ru0-nanoparticles for the transfer hydrogenation of substituted nitrobenzenes in IPA with retention of its activity for several cycles.11 A facile, simple and environment friendly hydrogen-transfer reaction over recyclable ferrite–nickel magnetic nanoparticles (Fe3O4–Ni) using glycerol as the hydrogen source affords the synthesis of aromatic amines and alcohols from nitroarenes and carbonyl compounds.12 Furthermore, ionic liquid supported Ni nanofibers are found to be highly efficient for reduction of substituted nitroarenes with NaBH4 at room temperature with >90% conversion within 2 h.13 On the other hand, oxide based heterogeneous catalysts have also been explored for CTH. For example, CeO2 nanorods exhibited excellent catalytic behaviour with high conversion and selectivity for a variety of aromatic nitro compounds using N2H4.14 Reduction of nitro derivatives to amines using IPA as a hydrogen source and KOH as a promoter is reported for a nano-catalyst MgO–ZrO2.15 Solid supported nano- and microparticles of Pd successfully convert nitroarenes to amines with various hydrogen donors.16
With increasing environmental concerns, much interest has been generated towards facile, sustainable and eco-friendly processes that include easily accessible raw materials, and simplicity in designing the catalysts that are efficient as well as inexpensive. To address this issue, we have selected iron based catalysts because of their low cost, nontoxicity and environmentally benign properties. The potentiality of iron based catalysts, either in bulk or nano-form, has been explored for reductive degradation of aquatic contaminants; however, their repeated use is restricted due to surface corrosion. By virtue of forming bimetallic species with other noble or non-noble metals, it is observed that such bimetallic systems remain active over longer periods due to the presence of other metal centers which eventually lead to increased catalytic efficiency and life-span of iron based catalysts. Keeping this view in mind, we have synthesized Fe–Ni NP's and employed them for transfer hydrogenation of nitroarenes with NaBH4 as the hydrogen source owing to its non-flammability, easily hydrolysable nature and controlled H2 production rate.17
2. Experimental
2.1. Chemicals
The following analytical grade chemicals were purchased from Loba Chemie, UK and used as received: iron(II) sulphate (FeSO4·7H2O), nickel(II) sulphate (NiSO4·6H2O), sodium borohydride (NaBH4). Deionized water is used throughout the experiments. All glassware and Teflon-coated magnetic stir bars are cleaned with acetone, followed by copious rinsing with distilled water before drying in an oven at 100 °C.2.2. Synthesis of Fe–Ni NP's
Fe–Ni NP's are synthesized in accordance with the procedure reported earlier.182.3. Characterization of nanocomposite
X-ray powder diffraction (XRD) patterns are recorded on a Phillips X'pert MPD X-ray diffractometer using Cu-Kα radiation. Transmission electron microscopic (TEM) images are obtained using a JEOL electron microscope (model 1200X) while X-ray photoelectron spectroscopy (XPS) analysis is carried out on a VG Micro Tech ESCA3000 instrument using Mg-Kα radiation (photo energy 1253.6 eV). Brunauer–Emmett–Teller (BET) surface area analysis is performed using the nitrogen adsorption method with surface analyzer system CHEMBET3000, Quantichrome Instruments, US. Kinetics of the transfer hydrogenation are monitored with a UV-Vis spectrometer (Lamda 25, Perkin Elmer) while the product analysis is carried out with GC-MS (Clarus 500, Perkin Elmer).2.4. Catalytic transfer hydrogenation
General method for the catalytic transfer hydrogenation of nitroarenes is as follows: in a 50 mL three neck round bottom flask, 1.6 mmoles of nitroarene is dissolved in 10 mL of methanol followed by addition of 0.16 mmoles of Fe–Ni NP's. The entire mixture is stirred at room temperature for 5 min and then 13 mmoles of NaBH4 dissolved in 5 mL of methanol is added drop wise with constant stirring till addition of NaBH4 is complete. The whole reaction mixture is further stirred for the desired period of time during which the progress of the reaction is monitored with TLC. After completion of the reaction, the catalyst is separated magnetically as well as by centrifugation (3000 rpm) and the separated reaction solution is subjected to GC-MS analysis. To investigate the reusability of Fe–Ni NP's, the magnetically separated catalyst is washed successively with acetone and vacuum-dried at room temperature before further catalytic cycles.3. Results and discussion
3.1. Characterization of Fe–Ni NP's
The XRD pattern of the Fe–Ni NP's (Fig. 1a) exhibits characteristic peaks corresponding to Fe–Ni alloy with diffraction patterns in the range of 40–65° that are assigned to (111) and (200) crystal planes of fcc (JCPDS 47-1417) as well as bcc (JCPDS 37-0474) Fe–Ni alloy.19 Additionally, the XRD peak at 40° can be ascribed to face centred cubic (fcc) of Fe–Ni alloy, which is consistent with the standard card (JCPDS card no. 38-0419). The morphology of Fe–Ni NP's (ESI; Fig. S1†) is found to be a spherical shape (20–40 nm) with surface area of 14.2 m2 g−1.3.2. Catalytic activity
It has been reported earlier that nitrobenzene is reduced by iron under anaerobic conditions to aniline in aqueous medium with nitrosobenzene as an intermediate product; however, a decreased reduction rate is attributed to the precipitation of siderite on the iron surface that inhibits further reduction processes.20 Although, hydrogenation of nitroarenes by nickel nanoparticles and hydrogen gas is ∼100%, such an approach is of limited use as these reactions are carried out at elevated temperatures (100 °C) that results in oxidation of the nickel surface.21 Moreover, nickel nanoparticles with isopropanol are proved to be suitable for chemoselective hydrogenation of ketones at 76 °C, however, formation of nickel oxide film on the surface of the catalyst consequently hampers its catalytic activity.22 Considering these shortcomings, we have adopted a rational approach wherein the corrosion of Fe0 or Ni0 is controlled through the formation of the bimetallic state that offers synergism on the surface and subsequently may enhance the reduction capabilities of the individual metals. In the present work, Fe–Ni NP's are employed as a heterogeneous catalyst in transfer hydrogenation of some substituted nitrobenzenes to the corresponding anilines (Table 1). Although there are several reports on the transfer hydrogenation of nitroarenes, these reactions are carried out at elevated temperatures as well as requiring higher reaction times either with noble or non-noble metals as heterogeneous catalysts.1,10 However, we have adopted a simple, facile and affordable route wherein Fe0 acts as an electron donor while Ni serves the purpose of electron shuttle thereby forming a galvanic cell on the bimetallic surface. Such a feature of the Fe–Ni bimetallic system has already been established for reductive degradation of organic and inorganic contaminants.18,23| Entry | Substrate | Product | Time | % Conversion | % Selectivity | TOFa × 10−4 (s−1) |
|---|---|---|---|---|---|---|
| a TOF = TON/reaction time. | ||||||
| 1 |  |
 |
35 min | 99.52 | 100 | 47.62 |
| 2 |  |
 |
4 h | 88.02 | 92 | 6.94 |
| 3 |  |
 |
5 h | 90.17 | 94 | 5.56 |
| 4 |  |
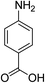 |
5 h | 95.57 | 98 | 5.56 |
| 5 |  |
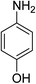 |
3 h | 99.37 | 100 | 9.26 |
| 6 | 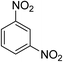 |
 |
6 h | 80.26 | 95 | 4.63 |
| 7 |  |
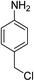 |
6 h | 99.79 | 100 | 4.63 |
| 8 |  |
 |
4 h | 98.85 | 100 | 6.94 |
| 9 |  |
 |
1 h | 99.57 | 100 | 2.78 |
| 10 | 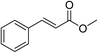 |
 |
1.2 h | 99.27 | 100 | 2.31 |
To optimise the amount of Fe–Ni NP's required for CTH with NaBH4, the nitrobenzene reaction is carried out at different catalyst loadings (ESI; Fig. S2†). It is observed that conversion is almost complete with 10 mol% which, therefore, is kept constant throughout the investigation. Table 1 illustrates the transfer hydrogenation of nitroarenes in the presence of Fe–Ni NP's carried out at room temperature with NaBH4 as the hydrogen source. It can be seen from this table that nitrobenzene (entry 1) is completely converted to aniline (∼100%) within 0.5 h with 100% selectivity (ESI; Fig. S3†). Interestingly, when either Fe0 or Ni0 nanoparticles are used for nitrobenzene reduction under identical conditions, only 50% conversion is observed with the former while reaction does not proceed using the latter catalyst (ESI Table T1†). To further access the rate of conversion, we have also established the kinetics of the hydrogenation of nitrobenzene catalyzed by Fe–Ni NP's in the presence of NaBH4 (Fig. 2a). It can be seen from this figure that reduction of nitrobenzene proceeds initially with a rapid decrease of reactant concentration up to about 97% but later on it follows almost first order reaction kinetics suggesting that the reaction is almost complete within 35 min. These findings clearly suggest that the catalytic activity is indeed due to both Fe and Ni nanoparticles in a bimetallic phase through synergistic effects on the catalytic surface.
 | ||
| Fig. 2 Time profile of nitrobenzene reduction for (a) fresh and (b) recovered Fe–Ni NP's. Inset shows magnetic separation of catalyst after completion of the reaction. | ||
The conversion of nitroarenes to corresponding amines is completed within a short span of time (0.5–6 h) with almost 98% conversion and about 95% selectivity. The lower selectivities of entries 2 and 3 are probably due to the presence of bulky methyl while m-dinitrobenzene (entry 6) is converted to the mono-amine with low yield (80%) and lower selectivity (Table 1). The latter conversion may be attributed to the strong desorption tendency of the formed basic m-nitroaniline on the negatively charged catalytic surface. On the other hand, selectivity for nitro-reduction is found to be ∼100% with almost 99% conversion for entries 7–9 implying that the catalyst possesses high selectivity towards the nitro-function. A similar facet is also observed for entry 10 where the double bond is selectively reduced and not the ester function. The plausible reason for such a facile conversion stems from the fact that the formation of metal hydride on the catalytic surface is facilitated by expulsion of hydrogen generated by borohydride in the reaction medium. Concurrently, the adsorption of nitroarene on the Fe–Ni NP's surface and simultaneous removal of a water molecule lead to formation of the nitroso compound, which subsequently combines with another molecule of hydrogen giving rise to hydroxylamine. Finally, hydroxylamine is reduced to the amine derivative in a slow reaction step with water as a byproduct (Scheme 1). It is also worth mentioning here that the turnover frequency (TOF) is 13 times higher for Fe-Ni NP's as compared to Pt–Ni bimetallic nanoparticles24 used for the transfer hydrogenation of nitroarenes under identical conditions. Thus, such a faster reduction process and considerably higher TOF is probably due to the efficient flow of electrons from Fe0 to the adjacent Ni0 site on the catalytic surface as well as the synergistic effect of both Fe–Ni and NaBH4 in tandem that plausibly enhances the liquid phase reduction process at room temperature.
3.3. Reusability studies
The efficiency of Fe–Ni NP's is further evaluated for repeated use without sacrificing the catalytic activity. For this purpose, transfer hydrogenation of nitrobenzene is carried out with recovered catalyst under identical experimental conditions. These results (Fig. 3) suggest that the catalyst remains highly active up to six cycles with >99% conversion for every cycle. Moreover, the reaction rate remains almost constant for the 1st cycle (2.31 × 10−3 s−1) up to the 6th cycle (2.25 × 10−3 s−1). Such a behavior can be explained on the basis of several aspects: (i) methanol (solvent) induces faster methanolysis of NaBH4 leading to rapid evolution of hydrogen25 that maintains nanoparticles well dispersed/suspended throughout the reduction, (ii) a small amount of liberated H2 expels air from the solution thereby preventing the surface corrosion of the catalyst, (iii) some amount of the liberated hydrogen also helps in retaining the zero valent state of the nanoparticles, (iv) formation of NaB(OCH3)4 during the methanolysis of NaBH4 facilitates continuous evolution of hydrogen and (v) sodium methoxide formed during the transfer hydrogenation reacts with NaBH4 which further enhances hydrogen generation. Moreover, to ascertain that the rate of conversion is not affected by successive usage of Fe–Ni NP's, we have established the kinetics of the hydrogenation of nitrobenzene catalyzed by recovered catalyst (Fig. 2b). It is interesting to note that catalytic activity remains almost the same even after the 6th cycle which suggests that its surface is not passivated even after exposure to several chemical treatments. Thus, all these factors contribute towards prevention of surface corrosion of the nanoparticle surface that consequently enhances its catalytic life. Such a feature has already been observed in the degradation of a dye by Fe–Ni NP's stored for 1 year; an orange G wherein it is observed that the reduction capacity is retained even after exposure to light and moisture.26 Hence, it can be argued that this bimetallic system is also efficient towards transfer hydrogenation of nitroarenes with high selectivity under simple laboratory conditions.These facts are further corroborated by the evaluation of the catalyst surface properties of Fe–Ni NP's before and after the transfer hydrogenation reduction of nitrobenzene. Techniques employed for this assessment include (i) XRD that assists in differentiating crystalline phases of Fe–Ni NP's and (ii) surface analysis by XPS which identifies the nature of the chemical species present on the surface of the Fe–Ni NP's before and after CTH. XRD analysis of these nanoparticles after the 6th cycle (Fig. 1b) indicates that there is no appreciable change in the pattern although the intensities of peaks are somewhat reduced. It is probably due to the successive exposure of these planes to the chemical environment that may lead to slight disturbances in the lattice structure. On the other hand, XPS analysis of nanoparticles after the last cycle was carried out for the iron and oxygen regions (Fig. 4) to examine the extent of surface corrosion that occurred on the nanoparticle surface. It can be seen that the nature and peak positions at 710, 712.4 and 715.3 eV for the iron region (Fig. 4a) remain almost identical with a slight change in their intensity pattern. Surprisingly, the amount of Fe(II) formed on the surface has increased by about 7% after the 6th cycle implying that some part of the liberated hydrogen from NaBH4 has also been utilized for the reduction of surface Fe(III) species. Such a surface phenomenon observed for Fe–Ni NP's, therefore, suggests that the surface corrosion is appreciably inhibited due to the presence of a reducing atmosphere during the entire course of the reaction. Moreover, these characteristics are also reflected in the oxygen region where the peaks at 530, 531.4 and 532.6 eV remain unchanged (Fig. 4b) even after the successive use of Fe–Ni NP's without much alteration in their intensities. Thus, it may be argued that almost 99% conversion of nitrobenzene to aniline with 100% selectivity is predominantly due to the existence of an anaerobic environment throughout six cycles and this approach may be regarded as a necessary feature for sustainable development in the field of catalytic transfer hydrogenation of organics without sacrificing the catalytic efficiency.
 | ||
| Fig. 4 XPS spectra of Fe–Ni NP's for (a) iron region and (b) oxygen region for fresh and recovered catalyst. | ||
3.4. Proposed mechanism
It is well known that metallic particles, either monometallic or multimetallic, being electronically conducting solids, strongly catalyze redox reactions. The proposed mechanism for the reduction of nitroarenes by borohydride in the presence of Fe–Ni NP's is illustrated in Scheme 1. The catalytic hydrogenation promoted by a H-donor proceeds on the surface of the metal nanoparticles. The generation of H2 on the catalyst surface is due to efficient methanolysis of sodium borohydride25 forming metal hydrides followed by intermolecular hydrogen transfer from the nanoparticle surface to adsorbed nitroarenes on the catalyst surface. Subsequently, loss of a water molecule leads to the formation of the nitroso compound, which being highly reactive, combines with another molecule of hydrogen giving rise to stable hydroxylamine. Since the conversion of hydroxylamine to aniline through dehydroxylation is the slower and rate determining step,3 variation in the conversion time is noted from 0.5–6 h for different nitroarenes. Water being the byproduct of CTH, it is possible that the iron surface may become corroded due to adsorbed water. However, in our case, the corrosion of the iron site is inhibited due to the presence of adjacent nickel sites as well as the anaerobic environment that prevents its surface passivation. The Ni sites also promote efficient flow of electrons and subsequently increase reduction rates and significantly enhance the catalytic activity of Fe–Ni NP's.4. Conclusions
The present investigation clearly demonstrates that nanoengineered Fe–Ni NP's indeed exhibit promising catalytic activity for transfer hydrogenation of nitroarenes under ambient conditions with almost 99% conversion having 100% selectivity. Since the iron surface is susceptible to corrosion, it may be argued that such high efficiency of the catalyst may be ascribed to synergism in Fe–Ni NP's that not only controls the iron passivation but also facilitates efficient flow of electron transfer from iron (donor) to nitroarenes (acceptor) mediated by Ni site that acts as an electron shuttle between donor and acceptor. This facet is further exemplified by their reusability potential where the catalyst surface remains active up to six cycles. To corroborate this aspect, XRD analysis of fresh and used catalysts suggests that the lattice structure remains almost the same even after repeated use. Moreover, surface properties are also not altered during the recycling process implying that the iron-corrosion process is effectively hindered due to adjacent Ni sites. It is also quite likely that some of the liberated H2 during CTH may be consumed by the catalyst to retain its activity. This catalytic system not only efficiently catalyzes the reduction of functionalized nitroarenes to the corresponding substituted anilines but also avoids the need for an inert atmosphere, and additional base or other additives. Thus, Fe–Ni NP's employed in the present investigation not only possess excellent catalytic activity with very high chemo-selectivity towards selective reduction of nitroarenes but they also can be magnetically separated from the reaction medium.Acknowledgements
Authors are thankful to Dr A. D. Natu Head, Dept. of Chemistry and Principal, A. G. College, for constant encouragement and support. DST, New Delhi is acknowledged for providing FIST grant.References
- P. Luo, K. Xu, R. Zhang, L. Huang, J. Wang, W. Xing and J. Huang, Catal. Sci. Technol., 2012, 2, 301–304 CAS.
- A. Tafesh and J. Weiguny, Chem. Rev., 1996, 96, 2035–2052 CrossRef CAS PubMed.
- H. Blaser, H. Steiner and M. Studer, ChemCatChem, 2009, 1, 210–221 CrossRef CAS.
- Y. Chen, N. Sasirekha and Y. Liu, J. Non-Cryst. Solids, 2009, 355, 1193–1201 CrossRef CAS PubMed.
- H. Liu, J. Deng and W. Li, Catal. Lett., 2010, 137, 261–266 CrossRef CAS.
- D. Guin, B. Baruwati and S. Manorama, Org. Lett., 2007, 9, 1419–1421 CrossRef CAS PubMed.
- V. Polshettiwar, B. Baruwati and R. Varma, Green Chem., 2009, 11, 127–131 RSC.
- S. Kidambi, J. Dai, J. Li and M. Bruening, J. Am. Chem. Soc., 2004, 126, 2658–2659 CrossRef CAS PubMed.
- Y. Niu, L. Yeung and R. Crooks, J. Am. Chem. Soc., 2001, 123, 6840–6846 CrossRef CAS.
- A. Saha and B. Ranu, J. Org. Chem., 2008, 73, 6867–6870 CrossRef CAS PubMed.
- P. Sarmah and D. Dutta, Green Chem., 2012, 14, 1086–1093 RSC.
- M. Gawande, A. Rathi, P. Branco, I. Nogueira, A. Velhinho, J. Shrikhande, U. Indulkar, R. Jayaram, C. Ghumman, N. Bundaleski and O. Teodoro, Chem.–Eur. J., 2012, 18, 12628–12632 CrossRef CAS PubMed.
- A. Chinnappan and H. Kim, RSC Adv., 2013, 3, 3399–3406 RSC.
- H. Zhu, Y. Lu, F. Fan and S. Yu, Nanoscale, 2013, 5, 7219–7223 RSC.
- M. Gawande, P. Branco, K. Parghi, J. Shrikhande, R. Pandey, C. Ghumman, N. Bundaleski, O. Teodoro and R. Jayaram, Catal. Sci. Technol., 2011, 1, 1653–1664 CAS.
- A. Shil, D. Sharma, N. Guha and P. Das, Tetrahedron Lett., 2012, 53, 4858–4861 CrossRef CAS PubMed.
- O. Ozay, N. Aktas, E. Inger and N. Sahiner, Int. J. Hydrogen Energy, 2011, 36, 1998–2006 CrossRef CAS PubMed.
- B. Kadu, Y. Sathe, A. Ingle, R. Chikate, K. Patil and C. Rode, Appl. Catal., B, 2011, 104, 407–414 CrossRef CAS PubMed.
- J. Xiang, X. Shen, F. Song, M. Liu, G. Zhou and Y. Chu, Mater. Res. Bull., 2011, 46, 258–261 CrossRef CAS PubMed.
- A. Agrawal and P. Tratnyek, Environ. Sci. Technol., 1996, 30, 153–160 CrossRef CAS.
- A. Wang, H. Yin, H. Lu, J. Xue, M. Ren and T. Jiang, Catal. Commun., 2009, 10, 2060–2064 CrossRef CAS PubMed.
- F. Alonso, P. Riente, J. Sirvent and M. Yus, Appl. Catal., A, 2010, 378, 42–51 CrossRef CAS PubMed.
- A. Bokare, R. Chikate, C. Rode and K. Paknikar, Appl. Catal., B, 2008, 79, 270–278 CrossRef CAS PubMed.
- S. Ghosh, M. Mandal, S. Kundu, S. Nath and T. Pal, Appl. Catal., A, 2004, 268, 61–66 CrossRef CAS PubMed.
- C. Lo, K. Karan and B. Davis, Ind. Eng. Chem. Res., 2009, 48, 5177–5184 CrossRef CAS.
- A. Bokare, R. Chikate, C. Rode and K. Paknikar, Environ. Sci. Technol., 2007, 41, 7437–7443 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: TEM image of Fe–Ni NP's, catalyst amount optimization, GC-MS data of CTH reactions and catalyst activity table. See DOI: 10.1039/c3ra45787g |
| This journal is © The Royal Society of Chemistry 2014 |




