LDA-promoted asymmetric synthesis of β-trifluoromethyl-β-amino indanone derivatives with virtually complete stereochemical outcome†
Abstract
We demonstrate that reactions between various 1-indanones and (SS)-N-tert-butanesulfinyl-(3,3,3)-trifluoroacetaldimine, conducted in the presence of catalytic amounts of LDA, occur with virtually complete stereochemical outcome, offering reliable and generalized access to biologically relevant β-trifluoromethyl-β-amino indanone derivatives. The products can be isolated in diastereomerically pure form simply by washing the crude reaction mixture with hexanes, underscoring practicality of the present method.
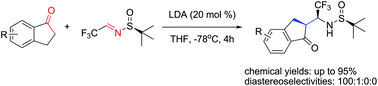
- This article is part of the themed collection: Organic chemistry collection

 Please wait while we load your content...
Please wait while we load your content...