Surface modification and partial reduction of three-dimensional macroporous graphene oxide scaffolds for greatly improved adsorption capacity†
Abstract
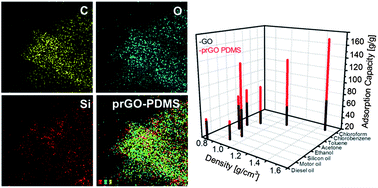
A surface modification and partial reduction of graphene oxide (GO) scaffolds by a coating of polymethylsiloxane (PDMS) leads to the production of an efficient pollutant adsorbent consisting of three-dimensional, macroporous, and hydrophobic prGO–PDMS architectures, resulting in great improvement of adsorption capacity by a factor of 3.53 and a reliable recyclability of ∼98% relative to the initial capacity.

 Please wait while we load your content...
Please wait while we load your content...