Fabrication of a CuInS2 photoelectrode using a single-step electrodeposition with controlled calcination atmosphere†
Abstract
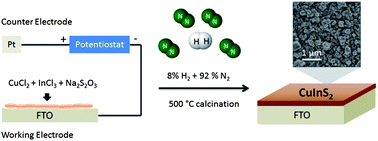
n-type CuInS2 thin films were fabricated by a one-step electrodeposition of Cu/In/S precursors on a transparent fluorine-doped tin oxide (FTO) substrate followed by calcination in a mixture of N2 and H2 at 500 °C without the need of toxic H2S gas for sulfurization. The analyses of structural and optical properties revealed that tetragonal chalcopyrite CuInS2 thin films were obtained when heat treated in a mixture of 8% H2 and 92% N2; whereas calcination in pure N2 resulted in the formation of CuInS2 with a considerable amount of In2O3 and CuxS impurities. The synthesized n-CuInS2 thin film has an optical bandgap of 1.3 eV with the conduction band at ca. −1.2 V vs. Ag/AgCl at pH 6.5. The heat treatment in a mixture of H2 and N2 provided a reducing atmosphere to suppress the oxide formation of the electrodeposited Cu/In/S elements. It also increased the donor density of CuInS2 sixfold by increasing the density of the sulfur vacancies (known to be electron donors) at the grain surface. As a result of the improved purity and increased donor density, the hydrogen treated CuInS2 thin film yielded a photocurrent density of ∼8 mA cm−2 at 0.5 V vs. Ag/AgCl in 0.25 M Na2S and 0.35 M Na2SO3 under visible light illumination, which is a fourfold enhancement as compared to that obtained from the CuInS2 film calcined in pure N2.

 Please wait while we load your content...
Please wait while we load your content...