Magnetic silica supported copper: a modular approach to aqueous Ullmann-type amination of aryl halides†
Abstract
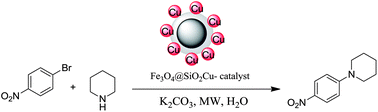
One-pot synthesis of a magnetic silica supported copper catalyst has been described via in situ generated magnetic silica (Fe3O4@SiO2); the catalyst can be used for the efficacious amination of aryl halides in aqueous medium under microwave irradiation.

 Please wait while we load your content...
Please wait while we load your content...