Mussel-inspired chemistry for one-step synthesis of N-doped carbon–gold composites with morphology tailoring and their catalytic properties†
Abstract
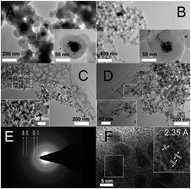
A coordination-assisted synthetic approach is reported for the synthesis of N-doped carbon supported highly active and stable gold catalysts starting with polydopamine-Au nanoparticles. The composites with tuning morphology via the control of the polymerization rate of dopamine could be used as efficient catalysts for reduction of 4-nitrophenol.

 Please wait while we load your content...
Please wait while we load your content...