Li-ion storage and gas adsorption properties of porous polyimides (PIs)†
Abstract
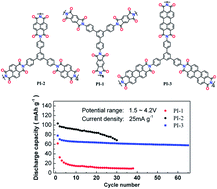
In this work, three polyimides (PIs) are successfully synthesized by the condensation reaction of an aromatic triamine with three difunctional aromatic dianhydrides. These materials show electrochemical lithium ion storage properties as cathodes for lithium-ion batteries. The aromatic dianhydride monomers are found to have significant effects on their performances. In addition, the polymer PI-1 shows narrow micropore size distributions and selective adsorption of CO2 over CH4 and N2. Furthermore, PI-1 exhibits a high Qst value (11.7 kJ mol−1) for H2 adsorption.

 Please wait while we load your content...
Please wait while we load your content...