Synthesis, structural properties, and pharmacological evaluation of 2-(acylamino)thiophene-3-carboxamides and analogues thereof†
Abstract
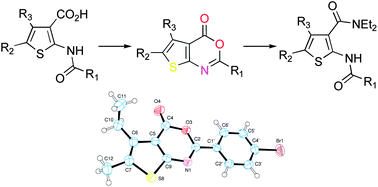
We have previously reported the synthesis and the pharmacological characterization of a family of methyl 2-(acylamino)thiophene-3-carboxylates as GABAB positive allosteric modulators active both in vitro and in vivo. In the present work, we describe the synthesis of new compounds based on the bioisosteric replacement of the ester moiety with amido or heterocyclic groups as well as of thieno[2,3-d]pyrimidine derivatives as rigid analogues thereof. 4H-Thieno[2,3-d][1,3]oxazin-4-ones were used as synthetic intermediates for the preparation of some of these compounds. The structures of oxazinones 11b, 16, and 17 were assigned by X-ray crystallographic studies, which definitely ruled out the isomeric beta-lactam structure previously hypothesized for these compounds. None of the new molecules exhibited significant activity at the GABAB receptor, either as allosteric or orthosteric ligands.

 Please wait while we load your content...
Please wait while we load your content...