Study on the microheterogeneity of aqueous alcohol solutions: formation mechanism of inner pores of ZnO nanostructures†
Abstract
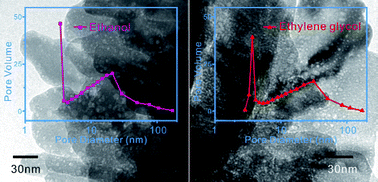
The self-association of alcohols in water has been demonstrated by a number of special experiments and theoretical work. Here, we report a simple and effective method to investigate the presence of these hydrophobic aggregation areas. Porous ZnO nanostructures were prepared using a simple alcohol assisted solution synthetic method at room temperature. The presence of hydrophobic groups of alcohols resulted in the existence of microheterogeneities and organic aggregations in the mixed solution, which act as a soft template and play key roles in the formation of the porous structure of ZnO. A high reaction temperature resulted in decreased hydrogen bonding between the alcohol and water molecules, and increased hydrophobic interactions among the alcohol molecules as well as increased pore sizes of the ZnO nanostructures. Sphere-like and hexagonal pores were observed, which were a result of the in situ enwrapping of hydrophobic aggregation areas in alcohol aqueous solution.

 Please wait while we load your content...
Please wait while we load your content...