Carbosilane cationic dendrimers synthesized by thiol–ene click chemistry and their use as antibacterial agents†
Elena
Fuentes-Paniagua
ab,
José Manuel
Hernández-Ros
c,
María
Sánchez-Milla
a,
M. Alejandra
Camero
a,
Marek
Maly
d,
Jorge
Pérez-Serrano
c,
José Luis
Copa-Patiño
c,
Javier
Sánchez-Nieves
ab,
Juan
Soliveri
c,
Rafael
Gómez
*ab and
F.
Javier de la Mata
*ab
aDepartamento de Química Orgánica y Química Inorgánica, Universidad de Alcalá, Campus Universitario, E-28871 Alcalá de Henares, Spain. E-mail: javier.delamata@uah.es
bNetworking Research Center on Bioengineering, Biomaterials and Nanomedicine (CIBER-BBN), Spain. E-mail: rafael.gomez@uah.es; Fax: +34 91 885 4683
cDepartamento de Biomedicina y Biotecnología, Universidad de Alcalá, Campus Universitario, E-28871, Alcalá de Henares, Spain. E-mail: juan.soliveri@uah.es
dFaculty of Science, J. E. Purkinje University, Ceske mladeze 8, 400 96 Usti n. L., Czech Republic
First published on 29th October 2013
Abstract
Cationic carbosilane dendrimers of generations 1–3 have been synthesized employing thiol–ene click chemistry. The obtained dendrimers present three different types of ammonium functions, two of them with the charge at the surface, –NH3+ and –NMe3+, and other with the charge internalized by the presence of ethylalcohol moieties, –[NMe2(CH2CH2OH)]+. The influence of –NMe3+ and –[NMe2(CH2CH2OH)]+ in dendrimer structure have been studied by molecular dynamics. The antibacterial properties of these families of dendrimers have been evaluated against Gram-positive (Staphylococcus aureus CECT 240) and Gram-negative (Escherichia coli CECT 515) bacterial strains, and the results have been compared with those obtained for related cationic carbosilane dendrimers functionalized by hydrosilylation reactions. These data show the relevance of the sulfur atom versus the silicon atom close to the dendrimer surface and the outer charge versus the inner charge. Finally, the stability of the most active first generation dendrimers vs. pH and temperature has also been studied.
1. Introduction
The increasing occurrences of microbial antibiotic resistance1 have prompted the search for alternatives to fight against infections. One of these alternatives is the use of quaternary ammonium salts (QAS), that have a broad spectrum of antimicrobial activity against both Gram-positive and Gram-negative bacteria.2 Their antibacterial activity is based on the fact that the majority of bacterial cell walls are negatively charged, and hence interaction between ammonium compounds and cell walls destroy them.3 Furthermore, other advantage of some QAS is their solubility in water, that allows them to kill bacteria in this medium.4 Another approach to avoid resistance is the use of polymeric multifunctional antimicrobials.5 The multivalency raises the activity of the functions attached to the macromolecule with respect to individual molecules. For biomedicine, this effect would lead to a stronger interaction of the macromolecule with its surrounding and this property has found a variety of applications.6–13 Other advantages of these systems when compared with small-molecule antimicrobial agents are non volatility, chemical stability, and long-term antimicrobial activity.14The combination of QAS with macromolecules generates polycations that present high superficial charge. Different types of these macromolecules such as polymers,14 dendrimers,15 and hyperbranched polymers16 have been studied as antimicrobial agents, exhibiting high antimicrobial activity due to their polyvalency. The activity and mechanism of antimicrobial polycations could be affected by several factors such as molecular weight, polydispersity, spacer length between active site and main scaffold, hydrophilic–hydrophobic balance, and nature of counterions.5,17
In our research group we have previously synthesized carbosilane dendrimers and hyperbranched polymers, which are interesting not only because of their chemical and thermal stability, but also for their inert framework and biocompatibility. We have studied their activity in different biomedical applications,18–20 including their antibacterial properties.21,22 However, we have found as main drawback of these systems the synthetic procedure to decorate the macromolecules, which involves several steps starting from the basic carbosilane olefin structures. First, hydrosilylation with chlorosilanes that often requires strictly controlled reaction conditions and are typically volatile and highly moisture sensitive, then substitution of Si–Cl bonds with Si–H bonds employing LiAlH4 and finally hydrosilylation of the required allylamine, with the inherent problems of this type of reactions. For these reasons, new functionalization routes that would allow keeping the main structure of these compounds while avoiding the most difficult steps of the synthetic procedure, have become necessary.
The reactions of sulfur containing compounds with alkenes known as “thiol–ene chemistry”23,24 are attractive due to their click characteristics,25 easy initiation, high-yields, minimal product purification, and high tolerance to a variety of solvents and functional groups. This methodology has been employed in polymer functionalization and macromolecular synthesis,25–29 including dendrimers.30–33 For example, Son and coworkers have reported the synthesis of carbosilane dendrimers via thiol–ene reactions34,35 and the functionalization of tetravinylsilane with a variety of thiols.36 Related carbosilane dendrimers were obtained previously by nucleophilic substitution of chloromethyl groups with thiolates by Krska and Seyferth.37
The aim of the present research was to assess the antimicrobial activities of new carbosilane dendrimers synthesized by thiol–ene click reactions. These new dendrimers present different types of cationic functions, –NH3+, –NMe3+, and another with the charge internalized by the presence of ethylalcohol moieties, –[NMe2(CH2CH2OH)]+. This last group was chosen to maintain the solubility of the carbosilane dendrimers, as the high hydrophobicity of the carbosilane framework would probably lead to very low or insoluble dendrimers if the charge is hidden from the surface. The structures of dendrimers with –NMe3+ and –[NMe2(CH2CH2OH)]+ groups were studied by molecular dynamics to determine any differences between both cationic functions that could affect their behaviour. The antibacterial properties of these systems were also studied and compared with related cationic dendrimers that present a Si atom close to the ammonium groups (i.e. the hydrosilylation approach)21,38 instead of S atoms (i.e. the thiol–ene approach). We believe that the incorporation of sulfur atoms close to the surface of the macromolecules would improve the antibacterial properties because it is known that natural and synthetic organosulfur compounds possess important antibiotic properties, being employed for treatment of microbial and parasitic infections.39–41
2. Results and discussion
2.1. Synthesis and characterization of dendrimers
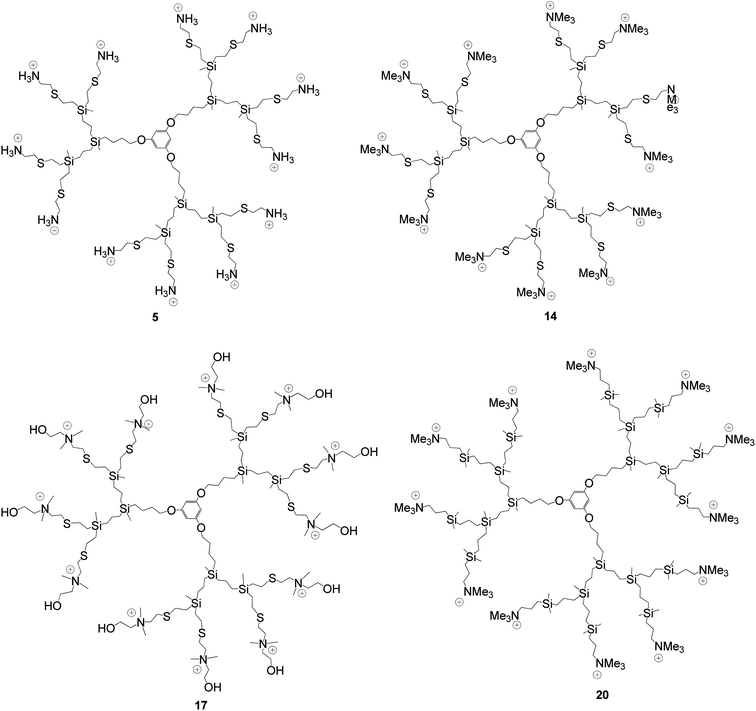
To carry out the thiol–ene reactions for obtaining the adequate cationic carbosilane dendrimers, we have first synthesized spherical dendrimers functionalized with vinyl groups at the periphery derived from 1,3,5-C6H3(OH)3. The procedure employed has been the same that the previously reported for analogues dendrimers with allyl groups,42 but using vinyl terminated dendrons43 that were coupled to the polyphenoxo core (see ESI†). The reason to introduce vinyl instead of allyl moieties was the higher reactivity of these groups toward the thiol–ene addition, as we have also observed in similar dendrons. These new compounds GnO3Vm (n = 1, m = 6 (1); n = 2, m = 12 (2); n = 3, m = 24 (3)) were characterized by NMR spectroscopy, MALDI-TOF and elemental analysis. The nomenclature employed for dendrimers described in this paper is of the type GnO3Xm, where Gn indicates generation, O3 core derived from 1,3,5-(HO)3C6H5, and Xm describe peripheral functions (X) and its number (m). The NMR spectra of the vinyl dendrimers 1–3 showed the characteristic signals of this group, two multiplets at δ 5.70 and 6.05 ppm in the 1H NMR (Fig. 1) and two resonances at δ 133.0 and 137.0 ppm in the 13C NMR spectra. In the 1H–29Si HMBC spectra, one crosspeak for the Si atom bound to the vinyl groups was observed at δ −13.5. On the other hand, very clean MALDI-TOF spectra were obtained for the 1–3 vinyl dendrimers, with fine peaks centered at m/z values matching those for the calculated proton adducts.Dendrimers decorated with ammonium functions were synthesized by thiol–ene reactions of the corresponding vinyl-terminated dendrimers GnO3Vm1–3 with commercially available aminoethanethiol hydrochloride or 2-(dimethylamino)ethanethiol hydrochloride (Scheme 1). The reactions were carried out under UV irradiation (365 nm) using a solvent mixture of THF–MeOH, a 1/1.2 thiol–alkene ratio, and with the presence of the radical initiator 2,2′-dimethoxy-2-diphenylacetophenone (DMPA) in 5% molar ratio with respect to thiol groups. After ca. 5 h. the new cationic carbosilane dendrimers GnO3(SNH3Cl)m (n = 1, m = 6 (4); n = 2, m = 12 (5); n = 3, m = 24 (6)) and GnO3(SNMe2·HCl)m (n = 1, m = 6 (7); n = 2, m = 12 (8); n = 3, m = 24 (9)) were obtained (Fig. 2). These systems 4–9 were isolated as white solids in high yields (over 80%), soluble in water and other polar solvents as alcohols and DMSO.
Structural characterization of dendrimers 4–9 has been carried out using elemental analysis, 1H, 13C, 15N and 29Si-NMR spectroscopy and mass spectrometry. The NMR spectroscopic and analytical data for derivatives 4–9 were consistent with their proposed structures (Fig. 2). The 1H and 13C-NMR spectra in DMSO showed for the carbosilane framework identical chemical shifts for analogous nuclei in different generations. The formation of the new branches –SiCH2CH2SCH2CH2N– was clearly shown in the 1H spectra by means of the triplets at δ ca. 0.87 and 2.60, for the inner methylene groups bonded to the silicon (a) and sulfur (b) atoms respectively, and by means of the triplets at δ ca. 2.76 and 2.94 (4–6) or 3.18 (7–9), for the outermost methylene groups bonded to the sulfur (c) and nitrogen (d) atoms. This observation was supported by data from COSY experiments. In the case of dendrimers 4–6 the protons directly bonded to the N atoms were observed as a broad singlet around δ 8.17, whereas for compounds 7–9 these protons appeared at δ ca. 11.1 and the methyl groups of the NMe2H groups were observed at δ ca. 2.74. The 13C{1H}-NMR spectra showed a signal around δ 41.3 corresponding to the methyl carbons of the NMe2H functions (e) (compounds 7–9) and a resonance at δ ca. 38.4 (4–6) or 55.2 (7–9) for the methylene bonded to the nitrogen atoms (d). Two signals were observed for the two different CH2S groups, one at δ ca. 24.5 and other at δ ca. 26.0 attributed to the external (c) and internal (b) methylene groups respectively (determined by 1H–13C-HMBC experiments), while the most external methylene carbon directly bonded to silicon atom (a) appears at δ ca. 13.6. The chemical shift of quaternized N atoms were detected in the 1H–15N NMR spectra by means of the resonances at δ ca. −342.4 (4–6) and at δ ca. −338.2 (7–9). 29Si NMR spectroscopy (1H–29Si-HMBC) clearly support the disappearance of the starting vinyl groups, appearing a new resonance at δ ca. 2.5 for the new outermost silicon atom Si(CH2)2S.
In order to synthesize dendrimers with cationic NMe3+ and [NMe2(CH2CH2OH)]+ moieties (Scheme 1), first we proceeded to neutralize dendrimers 7–9. Thus, addition of excess NaOH to these dendrimers allowed us to obtain the corresponding dimethylamine functionalized dendrimers GnO3(SNMe2)m (n = 1, m = 6 (10); n = 2, m = 12 (11); n = 3, m = 24 (12)). These compounds were isolated in good yield (over 80%) as pale yellow oils soluble in a variety of organic solvents, ethers, halogenated solvents, alcohols, DMSO, etc., but not in water. In general, the NMR data were very similar to those obtained for the parent compounds. The main differences were those related with the chemical change in the N atoms. In the 1H NMR spectra, the NMe2 and CH2N groups were shifted to lower frequency, δ ca. 2.25 and 2.55 respectively. The same behaviour was observed in the 13C NMR spectra for the C atoms of these groups (δ ca.45.2 and δ ca. 59.1) and for the chemical shift of the N atoms in the 1H–15N NMR spectra (δ ca. −352.1). However, the chemical shift of silicon atoms was not affected due to the distance to the N atoms.
From compounds 10–12 and by addition of excess MeI or HO(CH2)2I we have obtained the new ammonium-terminated dendrimers GnO3(SNMe3I)m (n = 1, m = 6 (13); n = 2, m = 12 (14); n = 3, m = 24 (15)) or GnO3[SNMe2(CH2CH2OH)I]m (n = 1, m = 6 (16); n = 2, m = 12 (17); n = 3, m = 24 (18)), respectively, in good yields (over 80%) as solids soluble in water, alcohols and DMSO (Fig. 2). For compounds GnO3(SNMe3I)m (13–15) the reaction was carried out at ambient temperature for 16 hours whereas for compounds GnO3(SNMe2(CH2CH2OH)I)m (16–18) heating at 60 °C for 3 days was necessary. Structural characterization of dendrimers 13–18 has been carried out using elemental analysis, 1H, 13C, 15N and 29Si-NMR spectroscopy and mass spectrometry.
The presence of the ammonium functions was confirmed in the 1H NMR spectra by the resonance corresponding to the methyl substituents of the NMe groups over δ 3.11 and in the 13C NMR spectra about δ 53.7, shifted to higher frequency with respect to the neutral dendrimers 10–12. The chemical shift corresponding to these N atoms was observed at δ ca. −330.0 (13–15) and δ ca. −324.2 (16–18), as consequence of the presence of the positive charge on the nitrogen atom. MS-ESI technique was used to determine [M − (mI)]m+ peaks of dendrimers GnO3(SNMe3I)m (13–15) and GnO3(SNMe2(CH2CH2OH)I)m (16–18) (see Experimental part).
2.2. Computer modeling
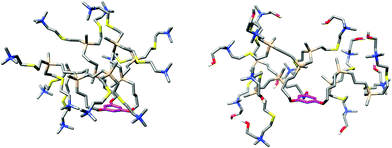
Computer models of the second generation cationic dendrimers 14 and 17 were created and simulated in explicit salt water using molecular dynamics in order to get detail information about their size and conformations in water environment, considering physical conditions: T = 310 K, P = 0.1 MPa. The most representative dendrimer structures from the last 30 ns of the total 65 ns long simulation have spherical shape with rather excentrically positioned core polyphenol ring (Fig. 3). Equilibrated dendrimer structures were consequently analyzed. Only slightly higher values of radius of gyration (Rg) and maximal distance of the dendrimer atoms from the dendrimer center of geometry (Rmax) were obtained for dendrimer 17 than for 14 (Rg = 10.0 Å (14), 10.6 Å (17); Rmax = 18.0 Å (14), 19.1 Å (17), as a consequence of the presence of the –(CH2)2OH chain.The radial distribution function (RDF) of both dendrimers (14, 17) is helpful to analyze more in detail and more clearly some differences between them (Fig. 4). The radial densities of N atoms of 14 and 17 have similar shapes and from ca. 8 Å are nearly identical. Both profiles have their maximum at 4.7 Å with 0.2 Å precision, which indicates presence of backfolding (Fig. S4, ESI†). It is more intensive in case of structure 17, as can be seen from the significantly higher maximal peak.
 | ||
| Fig. 4 Radial distribution density profiles of N and terminal O atoms (those from OH groups) with respect to C atoms from polyphenol ring. Numbers in parentheses denote given dendrimer. | ||
In the case of dendrimer 17, it is also interesting to compare the profiles of terminal N and O atoms. First of all, the O-profile has a significantly smaller maximal peak, in spite of the fact that each terminal oxygen atom is attached through just two-carbon spacer to the given nitrogen atom. However, this spacer gives to oxygen atoms some more degrees of freedom (comparing to N atoms) and this is reflected in a smaller maximal peak. On the other hand, the average density of terminal oxygen atoms in longer distances region, i.e. area of non-backfolded terminal units (from ca. 17.7 Å to ca. 22 Å), is higher than density of nitrogen atoms. This datum indicates that at least some oxygen atoms are not “backfolded” (carbon spacer is not bent), but that they rather form an outer portion of the molecular surface, being the –OH groups fully disposal for eventual interaction with another molecules (proteins, nucleic acids, etc.).
The effect of –NMe3+ and –[NMe2(CH2CH2OH)]+ groups in the external charge of dendrimers 14 and 17 was calculated and visualized by electrostatic potential on the molecular surface (Fig. 5). In the case of dendrimer 17 it is observed slightly more frequent red spots indicating lower potential values, due to the presence of the most electronegative oxygen atoms sufficiently close to the molecular surface. However, the effect of higher local electron density around oxygen atoms is of course not so high as, obviously, surrounding attached atoms have lower electron density. Also, the dipole character of –OH moieties can be detected on the molecular surface if they are oriented properly (Fig. S5, ESI†).
 | ||
| Fig. 5 Molecular surface of dendrimer models 14 (left) and 17 (right) colored according to electrostatic potential. The same conformations as those shown in Fig. 3 were used here. Top view (top), bottom view (bottom). Red color denotes low values (+5 kT/e (0.128 V) and lower) and the blue color means high potential values (+10 kT/e (0.256 V) and higher). The effect of water was implicitly taken into account in this electrostatic calculation. The yellow circle indicates position of polyphenol ring. | ||
To compare direct interaction of cationic terminal units of both dendrimers with anionic moieties we calculated also radial density profiles of Cl− anions with respect to N atoms (Fig. 6). In both dendrimers, the Cl− ion stabilized for a while by electrostatic interaction with the given cationic group is located at ca. 5 Å from the N atom. The noticeable higher maxima of dendrimer 14 might be connected mainly with better accessibility of the cationic charge (more suitable positions of Cl− anions at distance 5 Å from the central N atom are available) and eventually also with slightly stronger interaction due to smaller differences in local charge distribution than in dendrimer 17. Another difference between these compounds is that in the case of dendrimer 17 it is distinguished a second maxima (at ca. 6 Å) comparable with the first one, which involves interaction with the –OH dipole (Fig. S6, ESI†).
 | ||
| Fig. 6 Radial distribution density profiles of Cl− ions with respect to N atoms. Numbers in parentheses denote given dendrimer. | ||
2.3. Antibacterial activity
The biocide effect of cationic dendrimers is due to the high local concentration of cationic charges, and thus we have decided to compare the activity of the dendrimers with different outer modifications described above. For this purpose we have measured the growth of S. aureus CECT 240, as a model of Gram+ bacterias, and E. coli CECT 515, as a model of Gram− bacterias, in the presence and absence of dendrimer, in order to check the comparative activity of dendrimers to produce membrane disruption in these strains.The measuring of the minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) of these compounds (Table 1) have shown the influence of different parameters to take into account in the design of antibacterial polycationic compounds. First of all, we have compared the activity of cationic carbosilane dendrimers synthesized by thiol–ene reaction (13–15) with similar dendrimers obtained by hydrosilylation, GnO3(SiNMe3I)m (n = 1, m = 6 (19); n = 2, m = 12 (20); n = 3, m = 24 (21))38 (Fig. 2), in order to observe the influence of the sulfur atoms close to the dendritic periphery. In this sense, it has been found that the presence of the sulfur atom improves significantly the biocidal effect of these compounds, especially in the case of E. coli CECT 515 (Table 1). These results make the thiol derivatives 13–15 good candidates for this purpose, as disruption of Gram− bacterias is more complicated due to the presence of an extra layer in comparison to Gram+ bacterias, which just contain a thick peptidoglycan layer. It is important to highlight that biocidal activity decreases, in general, when dendritic generation increased. As biocidal activity of these types of compounds depends basically on two factors, the number of quaternized groups and the biopermeability processes, which depend on size and the presence of lipophilic domains, we can assume that for dendrimers 13–15 and 19–21 the second factor has more influence.
| Compound | 4 | 5 | 6 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | 21 | PenVK | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| S. aureus CECT 240 | MIC | 2 | 4 | 32 | 2 | 2 | 8 | 2 | 4 | 16 | 2 | 8 | 16 | 0.01 |
| MBC | 2 | 4 | 32 | 2 | 2 | 8 | 8 | 8 | 32 | 2 | 8 | 16 | 0.31 | |
| E. coli CECT 515 | MIC | 2 | 8 | 128 | 2 | 8 | 16 | 4–8 | 8 | 64 | 16 | 64 | 64 | 256 |
| MBC | 2 | 8 | 128 | 4 | 8 | 16 | 8 | 8 | 64 | 32 | 64 | 64 | 256 | |
On the other hand, to study the influence of nitrogen substituents, we have measured the biocidal activity of dendrimers that present different peripheral ammonium groups, as –NH3+ (4–6), –NMe3+ (13–15), and; –[NMe2(CH2CH2OH)]+ (16–18). In general, the charge internalization has a negative effect in the activity of these dendrimers (Table 1), indicating a diminishing of the interaction between dendrimers and bacterias, in accordance with theoretical data (see above) and with previously published results.44 The anomalous behaviour of third generation dendrimer 6 is attributed to solubility and aggregation problems.
The comparison of the data obtained from dendrimers with the antibiotic penicillin V potassium salt (PenVK, Table 1), an effective antibiotic against Gram-positive but not against Gram-negative bacteria, indicates that dendrimers are much more active against Gram-negative bacteria than penicillin, whereas their activity is similar against Gram-positive bacteria (this can be better observed when the MBC is measured as μM concentration, data don't shown). However, these systems can be considered as broad spectrum biocides, as the values found for the multivalent dendrimer systems are close for both type of bacterias.
One drawback of penicillins is their high instability against pH due to the presence of a β-lactam ring in their structure. For this reason we have performed a stability study of dendrimers toward variations of pH and temperature. For this purpose we have employed a response surface methodology using a three factor, three-level Box–Behnken design to analyze the effect of three variables in the stability of the dendrimers. The independent variables used in this design were: temperature (20–60 °C), pH (5–9 units pH) and incubation time of the dendrimer at different temperatures and pHs (6–48 hours). For this study we have chosen first generation compounds with –NMe3+ groups on their periphery obtained by thiol–ene click chemistry (13) and by hydrosilylation (19). We have discarded dendrimer 4, although it showed analogous activity, due to the presence of acid –NH3+ groups in its periphery that makes this compound instable in basic media, leading to the precipitation of a neutral dendrimer, G1O3(SNH2)6. This behaviour was confirmed by pH titration and the results compared with those obtained theoretically (see ESI†).
The stability study of compound 19 against pH, time and temperature showed that the activity of this compound is kept in all the measured conditions as is shown in Fig. 7 (near 100% growth inhibition). However, in the case of thio-ether dendrimer 13, its activity decreased in basic medium over 50 ºC. We believe that this is probably due to a very small decomposition of the outer functions due to Hofmann's elimination. As this process consists in the abstraction of a hydrogen atom from β-position with respect to the ammonium group by the hydroxide anion, the presence of a sulfur atom also in this β-position could probably enhance this decomposition. Unfortunately we have not been able to observe any modification of the NMR spectra of compound 13 when treated in these conditions, although any small change in the dendrimer surface may alter their antibacterial activity.
3. Conclusions
The combination of thiol–ene click reactions and quaternization with iodo-derivatives is a convenient methodology for the functionalization of carbosilane dendrimers with different ammonium groups. Thus, following this procedure we have synthesized cationic carbosilane dendrimers with –NH3+, –NMe3+, and, –[NMe2(CH2CH2OH)]+ groups.The structures of cationic dendrimers 14 and 17, with –NMe3+ and –[NMe2(CH2CH2OH)]+ groups respectively, were studied by computer modeling. The presence of hydroxyl groups in dendrimer 17 gives rise to slightly lower electrostatic potential on its surface and worse accessibility of the cationic charge. On the other hand, the OH dipoles can interact with anionic centers, so we can expect a better interaction of dendrimers 13–15 with proteins, as those that form part of bacterial membranes, and thus higher destabilization can be produced.
These cationic dendrimers and the analogous dendritic systems obtained through hydrosilylation with peripheral –NMe3+ moieties were studied as antimicrobial agents against Gram-positive (Staphylococcus aureus CECT 240) and Gram-negative (Escherichia coli CECT 515) bacterial strains. In general, the bactericide activity decreases with increasing generations. The results also showed a positive effect of the sulfur atom close to the surface with respect to those with a Si atom, being this effect more relevant in the case of Gram-negative bacterias, which are also more resistant bacterias to antibiotics due to the presence of a double layer membrane. With respect to the different types of ammonium groups, the activity decreases when the size of nitrogen substituents increases, as consequence of charge internalization that diminishes the interaction with the bacterial membrane. This supposition is in accordance with the results obtained from theoretical calculations. However, whereas the presence of a sulfur atom close to the nitrogen atoms enhances the antibacterial activity, this sulfur atom affects negatively the stability of thiol–ene dendrimers in basic medium at temperatures above 50 ºC.
Although the presence of the –OH moieties diminishes antibacterial activity, these groups could reinforced other type of interactions through hydrogen-bonding or also be used as a linker unit by formation of ester bonds with molecules of pharmacological interest. These possibilities are being now considered for ongoing studies.
4. Experimental section
4.1. General considerations
All reactions were carried out under inert atmosphere and solvents were purified from appropriate drying agents when necessary (THF). NMR spectra were recorded on a Varian Unity VXR-300 (300.13 (1H), 75.47 (13C) MHz) or on a Bruker AV400 (400.13 (1H), 100.60 (13C), 40.56 (15N), 79.49 (29Si) MHz). Chemical shifts (δ) are given in ppm. 1H and 13C resonances were measured relative to proton residues of deuterated solvent peaks considering TMS = 0 ppm, meanwhile 15N and 29Si resonances were measured relative to external MeNO and TMS, respectively. When necessary, assignment of resonances was done from HSQC, HMBC, COSY, TOCSY and NOESY NMR experiments. Elemental analyses were performed on a LECO CHNS-932. Mass Spectra were obtained from a Bruker Ultraflex III and an Agilent 6210. Thiol–ene reactions were carried out employing a HPK 125 W mercury lamp from Heraeus Noblelight with maximum energy at 365 nm, in normal glassware under an inert atmosphere. Compounds, HS(CH2)2NH2·HCl, HS(CH2)2NMe2·HCl, 2,2′-dimethoxy-2-phenylacetophenone (DMPA), MeI, crown ether 18-C-6, PenVK (Aldrich) and K2CO3 (Panreac) were obtained from commercial sources. Compounds GnO3(NMe3I)m (19–21) were synthesized as published.384.2. Synthesis of selected compounds
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 THF–methanol solution (5 mL). The reaction mixture was deoxygenated and irradiated for 1.5 h. Another 5% molar DMPA was added and the reaction mixture was irradiated for another 1.5 h and monitored by 1H-NMR. The initial reaction mixture was concentrated via rotator evaporation and solved in water. Then, nanofiltration with membranes of MW = 500 was performed. The pure product was dried in vacuo to afford 4 as a white solid (0.140 g, 60%). Data for 4: NMR (DMSO): 1H-NMR: δ −0.02 (s, 9H, SiMe), 0.61 (t, 6H, OCH2CH2CH2CH2SiMe), 0.87 (t, 12H, –SiCH2CH2S), 1.40 (m, 6H, OCH2CH2CH2CH2SiMe), 1.69 (m, 6H, OCH2CH2CH2CH2SiMe), 2.75 (t, 12H, –SiCH2CH2S), 2.77 (t, 12H, SCH2CH2NH3+), 2.93 (t, 12H, SCH2CH2NH3+), 3.90 (t, 6H, OCH2CH2CH2CH2SiMe), 6.03 (s, 3H, C6H3O3), 8.10 (s, 3H, –NH3+). 13C-NMR: δ −5.4 (SiMe), 12.7 (OCH2CH2CH2CH2SiMe), 13.9 (–SiCH2CH2S), 19.7 (OCH2CH2CH2CH2SiMe), 26.3 (–SiCH2CH2S), 27.7 (SCH2CH2NH3+), 32.4 (OCH2CH2CH2CH2SiMe), 38.4 (SCH2CH2NH3+), 67.0 (OCH2CH2CH2CH2SiMe), 93.6 (C6H3O3, C–H), 160.5 (C6H3O3, C–O). 15N-NMR: δ −342.4 (–NH3+). 29Si-NMR: 2.1 (SiMe). ESI: (1260.38 g mol−1) q = 2 (523.28 [M − 4HCl − 2Cl−]2+), q = 3 (349.18 [M − 3HCl − 3Cl−]3+). Anal. calcd C45H102Cl6N6O3S6Si3 (1264.69 g mol−1): C, 42.74; H, 8.13; N, 6.65; S, 15.21. Exp.: C, 39.21; H, 7.61; N, 6.42; S, 15.56.
2 THF–methanol solution (5 mL). The reaction mixture was deoxygenated and irradiated for 1.5 h. Another 5% molar DMPA was added and the reaction mixture was irradiated for another 1.5 h and monitored by 1H-NMR. The initial reaction mixture was concentrated via rotator evaporation and solved in water. Then, nanofiltration with membranes of MW = 500 was performed. The pure product was dried in vacuo to afford 4 as a white solid (0.140 g, 60%). Data for 4: NMR (DMSO): 1H-NMR: δ −0.02 (s, 9H, SiMe), 0.61 (t, 6H, OCH2CH2CH2CH2SiMe), 0.87 (t, 12H, –SiCH2CH2S), 1.40 (m, 6H, OCH2CH2CH2CH2SiMe), 1.69 (m, 6H, OCH2CH2CH2CH2SiMe), 2.75 (t, 12H, –SiCH2CH2S), 2.77 (t, 12H, SCH2CH2NH3+), 2.93 (t, 12H, SCH2CH2NH3+), 3.90 (t, 6H, OCH2CH2CH2CH2SiMe), 6.03 (s, 3H, C6H3O3), 8.10 (s, 3H, –NH3+). 13C-NMR: δ −5.4 (SiMe), 12.7 (OCH2CH2CH2CH2SiMe), 13.9 (–SiCH2CH2S), 19.7 (OCH2CH2CH2CH2SiMe), 26.3 (–SiCH2CH2S), 27.7 (SCH2CH2NH3+), 32.4 (OCH2CH2CH2CH2SiMe), 38.4 (SCH2CH2NH3+), 67.0 (OCH2CH2CH2CH2SiMe), 93.6 (C6H3O3, C–H), 160.5 (C6H3O3, C–O). 15N-NMR: δ −342.4 (–NH3+). 29Si-NMR: 2.1 (SiMe). ESI: (1260.38 g mol−1) q = 2 (523.28 [M − 4HCl − 2Cl−]2+), q = 3 (349.18 [M − 3HCl − 3Cl−]3+). Anal. calcd C45H102Cl6N6O3S6Si3 (1264.69 g mol−1): C, 42.74; H, 8.13; N, 6.65; S, 15.21. Exp.: C, 39.21; H, 7.61; N, 6.42; S, 15.56.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 THF–methanol solution (3 mL) using the preparative procedure for 4. The pure product was dried in vacuo to afford 7 as a white solid (0.533 g, 58%). Data for 7: NMR (DMSO): 1H-NMR: δ 0.03 (s, 9H, SiMe), 0.61 (m, 6H, OCH2CH2CH2CH2Si), 0.88 (t, Ja = 8.3 Hz, 12H, SiCH2CH2S), 1.40 (m, 6H, OCH2CH2CH2CH2Si), 1.69 (m, 6H, OCH2CH2CH2CH2Si), 2.61 (t, Ja = 8.7 Hz, 12H, SiCH2CH2S), 2.71 (s, 36H, SCH2CH2NMe2H+), 2.86 (t, Jb = 7.9 Hz, 12H, SCH2CH2NMe2H+), 3.22 (t, Ja = 8.0 Hz, 12H, SCH2CH2NMe2H+), 3.90 (m, 6H, OCH2), 6.03 (s, 3H, C6H3O3), 10.71 (bs, 6H, –NMe2H+). 13C-NMR: δ −5.8 (SiMe), 12.2 (OCH2CH2CH2CH2Si), 13.5 (SiCH2CH2S), 19.3 (OCH2CH2CH2CH2Si), 24.2 (SCH2CH2NMe2H+), 26.0 (SiCH2CH2S), 32.0 (OCH2CH2CH2CH2Si), 41.4 (SiCH2CH2NMe2H+), 55.3 (SCH2CH2NMe2H+), 66.5 (OCH2), 93.1 (C6H3O3; C–H), 159.9 (C6H3O3; C–O). 15N-NMR: δ −338.3 (-NMe2H+). 29Si-NMR: δ 3.1 (G1–SiMe). Anal. calcd C57H126Nj6Cl6O3S6Si3 (1433.01 g mol−1): C, 47.77; H, 8.86; N, 5.86; S, 13.43; found: C, 46.62; H, 8.76; N, 5.90; S, 12.63.H.
2 THF–methanol solution (3 mL) using the preparative procedure for 4. The pure product was dried in vacuo to afford 7 as a white solid (0.533 g, 58%). Data for 7: NMR (DMSO): 1H-NMR: δ 0.03 (s, 9H, SiMe), 0.61 (m, 6H, OCH2CH2CH2CH2Si), 0.88 (t, Ja = 8.3 Hz, 12H, SiCH2CH2S), 1.40 (m, 6H, OCH2CH2CH2CH2Si), 1.69 (m, 6H, OCH2CH2CH2CH2Si), 2.61 (t, Ja = 8.7 Hz, 12H, SiCH2CH2S), 2.71 (s, 36H, SCH2CH2NMe2H+), 2.86 (t, Jb = 7.9 Hz, 12H, SCH2CH2NMe2H+), 3.22 (t, Ja = 8.0 Hz, 12H, SCH2CH2NMe2H+), 3.90 (m, 6H, OCH2), 6.03 (s, 3H, C6H3O3), 10.71 (bs, 6H, –NMe2H+). 13C-NMR: δ −5.8 (SiMe), 12.2 (OCH2CH2CH2CH2Si), 13.5 (SiCH2CH2S), 19.3 (OCH2CH2CH2CH2Si), 24.2 (SCH2CH2NMe2H+), 26.0 (SiCH2CH2S), 32.0 (OCH2CH2CH2CH2Si), 41.4 (SiCH2CH2NMe2H+), 55.3 (SCH2CH2NMe2H+), 66.5 (OCH2), 93.1 (C6H3O3; C–H), 159.9 (C6H3O3; C–O). 15N-NMR: δ −338.3 (-NMe2H+). 29Si-NMR: δ 3.1 (G1–SiMe). Anal. calcd C57H126Nj6Cl6O3S6Si3 (1433.01 g mol−1): C, 47.77; H, 8.86; N, 5.86; S, 13.43; found: C, 46.62; H, 8.76; N, 5.90; S, 12.63.H.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 20 mL) solution of 7 (0.265 g, 0.18 mmol), a NaOH solution was added drop by drop (0.055 g, 1.38 mmol). The reaction mixture was stirred for 15 minutes at room temperature, and finally the aqueous phase was removed. The organic phase was dried in vacuo to get 10 as a pale yellow oil (0.224 g, 100%). Data for 10: NMR (CDCl3): 1H-NMR: δ 0.01 (s, 9H, SiMe), 0.60 (m, 6H, OCH2CH2CH2CH2Si), 0.90 (t, J = 8.6 Hz, 12H, SiCH2CH2S), 1.45 (m, 6H, OCH2CH2CH2CH2Si), 1.75 (m, 6H, OCH2CH2CH2CH2Si), 2.25 (s, 36H, SCH2CH2NMe2), 2.47 (m, 12H, SCH2CH2NMe2), 2.52 (m, 12H, SiCH2CH2S), 2.59 (m, 12H, SCH2CH2NMe2), 3.85 (t, J = 6.3 Hz, 6H, OCH2), 6.02 (s, 3H, C6H3O3). 13C-NMR: δ −5.4 (SiMe), 13.3 (OCH2CH2CH2CH2Si), 14.5 (SiCH2CH2S), 20.3 (OCH2CH2CH2CH2Si), 27.6 (SCH2CH2NMe2), 29.8 (SiCH2CH2S), 33.0 (OCH2CH2CH2CH2Si), 45.4 (SiCH2CH2NMe2), 59.2 (SCH2CH2NMe2), 67.3 (OCH2), 93.7 (C6H3O3; C–H), 160.8 (C6H3O3; C–O). 15N-NMR: δ −352.1 (–NMe2). 29Si-NMR: δ 2.4 (G1–SiMe). MS: [M + H]+ = 1213.7 uma (calcd = 1213.8 uma). Anal. calcd C57H120N6O3S6Si3 (1214.25 g mol−1): C, 56.38; H, 9.96; N, 6.92; S, 15.84; found: C, 55.68; H, 9.43; N, 6.77; S, 14.94.
1, 20 mL) solution of 7 (0.265 g, 0.18 mmol), a NaOH solution was added drop by drop (0.055 g, 1.38 mmol). The reaction mixture was stirred for 15 minutes at room temperature, and finally the aqueous phase was removed. The organic phase was dried in vacuo to get 10 as a pale yellow oil (0.224 g, 100%). Data for 10: NMR (CDCl3): 1H-NMR: δ 0.01 (s, 9H, SiMe), 0.60 (m, 6H, OCH2CH2CH2CH2Si), 0.90 (t, J = 8.6 Hz, 12H, SiCH2CH2S), 1.45 (m, 6H, OCH2CH2CH2CH2Si), 1.75 (m, 6H, OCH2CH2CH2CH2Si), 2.25 (s, 36H, SCH2CH2NMe2), 2.47 (m, 12H, SCH2CH2NMe2), 2.52 (m, 12H, SiCH2CH2S), 2.59 (m, 12H, SCH2CH2NMe2), 3.85 (t, J = 6.3 Hz, 6H, OCH2), 6.02 (s, 3H, C6H3O3). 13C-NMR: δ −5.4 (SiMe), 13.3 (OCH2CH2CH2CH2Si), 14.5 (SiCH2CH2S), 20.3 (OCH2CH2CH2CH2Si), 27.6 (SCH2CH2NMe2), 29.8 (SiCH2CH2S), 33.0 (OCH2CH2CH2CH2Si), 45.4 (SiCH2CH2NMe2), 59.2 (SCH2CH2NMe2), 67.3 (OCH2), 93.7 (C6H3O3; C–H), 160.8 (C6H3O3; C–O). 15N-NMR: δ −352.1 (–NMe2). 29Si-NMR: δ 2.4 (G1–SiMe). MS: [M + H]+ = 1213.7 uma (calcd = 1213.8 uma). Anal. calcd C57H120N6O3S6Si3 (1214.25 g mol−1): C, 56.38; H, 9.96; N, 6.92; S, 15.84; found: C, 55.68; H, 9.43; N, 6.77; S, 14.94.
4.3. Computational methods
Computer atomistic models of dendrimers 14 and 17 were created (using Materials Studio software package from Accelrys Inc.) and parametrized. All dendrimers were consequently simulated in salt explicit water (TIP3P water model, 0.15 M of NaCl) for 65 ns under conditions T = 310 K and P = 0.1 MPa using Molecular Dynamics. All the equilibrated dendrimer structures from the last 30 ns of simulations were consequently analysed in terms of their size and density distribution of selected molecular components (radial distribution function). Electrostatic potential on molecular surface of the most representative dendrimer conformations was calculated/visualized (Adaptive Poisson–Boltzmann Solver, VMD). Please see the ESI for more details.†4.4. pH titration
A solution of NaOH (1.8 μM) was carefully added to a solution of 4 (38.5 mg) in water. The pH was measured using a pH-Meter Basic 20+ from Crison.4.5. Antibacterial methodology
The minimal inhibitory concentration (MIC) of the products was measured in 96-well tray microplates using the international standard methods ISO 20776-1 by microdilution tray preparations.45 The assays were done in duplicate microplates with three different wells for each concentration analyzed in the microplate. The bacteria used in the analysis were Escherichia coli (CECT 515) (Gram-negative) and Staphylococcus aureus (CECT 240) (Gram-positive). Both strains were obtained from the “Colección Española de cultivos tipo” (CECT). A stock solution of the products was obtained by dissolving 0.01024 g of the compound with 10 mL of distilled water. After that, distilled water was added to obtain the desired concentration. The microplates were incubated at 37 °C using an ultra microplate reader ELX808iu (Bio-Tek Instruments). The minimal bactericidal concentration (MBC) was calculated by inoculating 3 μL of the samples used to calculate the MIC in Petri dish with Mueller-Hinton agar. The samples were put on a drop in the plates. After 48 h of incubation at 37 °C the presence of colonies was tested. The MBC was the minimal concentration in which not growth was detected.For the stability experiments, the growth inhibition of the microorganisms was measured after the dendrimer was incubated in the different conditions. The software Statgraphics Centurion XV was used to make the Box–Behnken design.
Acknowledgements
This work has been supported by grants from CTQ2011-23245 (MINECO), and Consortium NANODENDMED ref S2011/BMD-2351 (CM) to University of Alcalá. This work was also supported by grants from the Ministerio de Educación for E.F.P. CIBER-BBN is an initiative funded by the VI National R&D&i Plan 2008–2011, Iniciativa Ingenio 2010, Consolider Program, CIBER Actions and financed by the Instituto de Salud Carlos III with assistance from the European Regional Development Fund.References
- A. Coates, Y. M. Hu, R. Bax and C. Page, Nat. Rev. Drug Discov., 2002, 1, 895–910 CrossRef CAS PubMed.
- M. Tischer, G. Pradel, K. Ohlsen and U. Holzgrabe, Chem. Med. Chem., 2012, 7, 22–31 CrossRef CAS PubMed.
- E. R. Kenawy, F. I. Abdel-Hay, A. E. R. R. El-Shanshoury and M. H. El-Newehy, J. Polym. Sci., Part A: Polym. Chem., 2002, 40, 2384–2393 CrossRef CAS.
- R. B. Tompkin, Food Technol., 1984, 38, 226 Search PubMed.
- A. Muñoz-Bonilla and M. Fernández-García, Prog. Polym. Sci., 2012, 37, 281–339 CrossRef PubMed.
- X. Liu, P. Rocchi and L. Peng, New J. Chem., 2012, 9, 256–263 RSC.
- J. L. Jiménez, M. Pion, F. J. de la Mata, R. Gómez, E. Muñoz, M. Leal and M. A. Muñoz-Fernández, New J. Chem., 2012, 36, 299–309 RSC.
- A. J. L. Villaraza, A. Bumb and M. W. Brechbiel, Chem. Rev., 2010, 110, 2921–2959 CrossRef CAS PubMed.
- P. M. H. Heegaard, U. Boas and N. S. Sorensen, Bioconjugate Chem., 2010, 21, 405–418 CrossRef CAS PubMed.
- S. Svenson, Eur. J. Pharm. Biopharm., 2009, 71, 445–462 CrossRef CAS PubMed.
- D. G. Shcharbin, B. Klajnert and M. Bryszewska, Biochemistry, 2009, 74, 1070–1079 CAS.
- O. Rolland, C. O. Turrin, A. M. Caminade and J. P. Majoral, New J. Chem., 2009, 33, 1809–1824 RSC.
- N. Joshi and M. Grinstaff, Curr. Top. Med. Chem., 2008, 8, 1225–1236 CrossRef CAS.
- B. Dizman, M. O. Elasri and L. J. Mathias, J. Appl. Polym. Sci., 2004, 94, 635–642 CrossRef CAS.
- S. Hou, C. Zhou, Z. Liu, A. W. Young, Z. Shi, D. Ren and N. R. Kallenbach, Bioorg. Med. Chem. Lett., 2009, 19, 5478–5481 CrossRef CAS PubMed.
- T. Osamura, M. Ozawa and T. Iwama, Patent JP2011037716A, 2011.
- T. Ikeda, H. Hirayama, H. Yamaguchi, S. Tazuke and M. Watanabe, Antimicrob. Agents Chemother., 1986, 30, 132–136 CrossRef CAS.
- J. L. Jiménez, M. I. Clemente, N. D. Weber, J. Sánchez, P. Ortega, F. J. de la Mata, R. Gómez, D. García, L. A. López-Fernández and M. A. Muñoz-Fernández, Biodrugs, 2010, 24, 331–343 CrossRef PubMed.
- I. Posadas, B. López-Hernández, M. I. Clemente, J. L. Jiménez, P. Ortega, J. de la Mata, R. Gómez, M. A. Muñoz-Fernández and V. Ceña, Pharm. Res., 2009, 26, 1181–1191 CrossRef CAS PubMed.
- J. F. Bermejo, P. Ortega, L. Chonco, R. Eritja, R. Samaniego, M. Muellner, E. de Jesús, F. J. de la Mata, J. C. Flores, R. Gómez and A. Muñoz-Fernández, Chem. Eur. J., 2007, 13, 483–495 CrossRef CAS PubMed.
- P. Ortega, M. Cobaleda, J. M. Hernández-Ros, E. Fuentes-Paniagua, J. Sánchez-Nieves, M. P. Tarazona, J. L. Copa-Patiño, J. Soliveri, F. J. de la Mata and R. Gómez, Org. Biomol. Chem., 2011, 9, 5238–5248 CAS.
- B. Rasines, J. M. Hernández-Ros, N. de las Cuevas, J. L. Copa-Patiño, J. Soliveri, M. A. Muñoz-Fernández, R. Gómez and F. J. de la Mata, Dalton Trans., 2009, 8704–8713 RSC.
- M. J. Kade, D. J. Burke and C. J. Hawker, J. Polym. Sci., Part A: Polym. Chem., 2010, 48, 743–750 CrossRef CAS.
- B. H. Northrop and R. N. Coffey, J. Am. Chem. Soc., 2012, 134, 13804–13817 CrossRef CAS PubMed.
- C. E. Hoyle and C. N. Bowman, Angew. Chem., Int. Ed., 2010, 49, 1540–1573 CrossRef CAS PubMed.
- A. B. Love, Polym. Chem., 2010, 1, 17–36 RSC.
- J. Mergy, A. Fournier, E. Hachet and R. Auzely-Velty, J. Polym. Sci., Part A: Polym. Chem., 2012, 50, 4019–4028 CrossRef CAS.
- V. Darcos, S. Antoniacomi, C. Paniagua and J. Coudane, Polym. Chem., 2012, 3, 362–368 RSC.
- C. Rissing and D. Y. Son, Main Group Chem., 2009, 8, 251–262 CrossRef CAS.
- K. L. Killops, L. M. Campos and C. J. Hawker, J. Am. Chem. Soc., 2008, 130, 5062–5064 CrossRef CAS PubMed.
- T. Kang, R. J. Amir, A. Khan, K. Ohshimizu, J. N. Hunt, K. Sivanandan, M. I. Montáñez, M. Malkoch, M. Ueda and C. J. Hawker, Chem. Commun., 2010, 46, 1556–1558 RSC.
- M. I. Montáñez, L. M. Campos, P. Antoni, Y. Hed, M. V. Walter, B. T. Krull, A. Khan, A. Hult, C. J. Hawker and M. Malkoch, Macromolecules, 2010, 43, 6004–6013 CrossRef.
- C. Nilsson, E. Malmström, M. Johansson and S. M. Trey, J. Polym. Sci., Part A: Polym. Chem., 2009, 47, 589–601 CrossRef CAS.
- C. Rissing and D. Y. Son, Organometallics, 2009, 28, 3167–3172 CrossRef CAS.
- L. Chen, T. E. Andersson, C. Rissing, S. Yang, S. Chen and D. Y. Son, J. Mater. Chem. B, 2013, 1, 116–122 RSC.
- C. Rissing and D. Y. Son, Organometallics, 2008, 27, 5394–5397 CrossRef CAS.
- S. W. Krska and D. Seyferth, Organometallics, 1998, 120, 3604–3612 CAS.
- E. Pedziwiatr-Werbicka, E. Fuentes-Paniagua, V. Dzmitruk, J. Sánchez-Nieves, M. Sudas, E. Drozd, A. Shakhbazau, D. Shcharbin, F. J. de la Mata, R. Gómez-Ramírez, M. Á. Muñoz-Fernández and M. Bryszewska, Colloids Surf., B, 2013, 109, 183–189 CrossRef CAS PubMed.
- B. Heldreth and E. Turos, Curr. Med. Chem., 2005, 4, 295–315 CAS.
- S. Kim, R. Kubec and R. A. Musah, J. Ethnopharmacol., 2006, 104, 188–192 CrossRef CAS PubMed.
- H. Tapiero, D. M. Townsend and K. D. Tew, Biomed. Pharmacother., 2004, 58, 183–193 CrossRef CAS PubMed.
- J. Sánchez-Nieves, P. Ortega, M. Á. Muñoz-Fernández, R. Gómez and F. J. de la Mata, Tetrahedron, 2010, 66, 9203–9213 CrossRef PubMed.
- E. Fuentes-Paniagua, C. E. Peña-González, M. Galán, R. Gómez, F. J. d. l. Mata and J. Sánchez-Nieves, Organometallics, 2013, 32, 1789–1796 CrossRef CAS.
- C. Z. Chen, N. C. Beck-Tan, P. Dhurjati, T. K. van Dyk, R. A. LaRossa and S. L. Cooper, Biomacromolecules, 2000, 1, 473–480 CrossRef CAS.
- Reference Methods for the testing the “in vitro” activity of antimicrobial agents against bacteria involved in infectious diseases (ISO 20776-1), 2006 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: pH titration experiment of dendrimer 4 and theoretical calculation, description of synthesis of second and third generation dendrimers, selected NMR spectra. See DOI: 10.1039/c3ra45408h |
| This journal is © The Royal Society of Chemistry 2014 |