Electro-actuation of biocompatible Pluronic/methacrylic acid hydrogel in blood-plasma and in blood-mimicking buffers†
Abstract
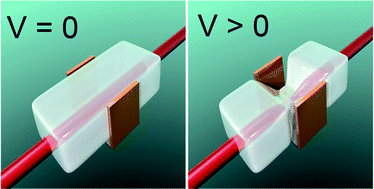
The electro-responsiveness in blood plasma and in blood mimicking fluids of methacrylic acid modified Pluronic (P127) hydrogel is investigated. It is observed that the hydrogel's response to an applied potential, in all buffer solutions, is very high, and comparable in amplitude and time response to classic polyelectrolyte gels. The highest actuation amplitude is achieved in PBS, which is directly related to the largest current value obtained for that buffer. The electro-actuation achieved in blood plasma is comparable to that in KREBS and KCl buffers. The good performance in blood plasma is attributed to the low protein adhesion to hydrogel surface. Preliminary biocompatibility studies demonstrate that the investigated hydrogel can be considered biocompatible.

 Please wait while we load your content...
Please wait while we load your content...