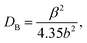Controlled nucleation and growth of nanostructures by employing surface modified GaN based layers/heterostructures as bottom layer
R. Ramesh,
R. Loganathan,
Sumithra Sivadas Menon,
K. Baskar and
Shubra Singh*
Crystal Growth Centre, Anna University, Chennai, 600 025, India. E-mail: shubra6@gmail.com
First published on 3rd January 2014
Abstract
Controlled nucleation and growth of Zinc oxide nanorods is achieved on GaN, etched GaN and AlGaN/GaN heterostructure bottom layers grown by a metal organic chemical vapour deposition technique. The effects of the bottom crystalline layers on the structural, morphological and optical properties of the as grown ZnO nanorods have been investigated by high-resolution X-ray diffraction, scanning electron microscopy, photoluminescence and Raman measurements. HRXRD (0002) reciprocal-space mapping (RSMs) studies were performed on GaN and AlGaN/GaN layers before and after the growth of ZnO nanostructures to investigate the impact of strain upon the ZnO layer grown on GaN layers and AlGaN/GaN heterostructures. Raman intensity mapping shows the densely nucleated hexagonal pit like structures for the etched GaN layer, providing an enhanced surface area for primary nucleation suggesting that the growth species prefer to condense on locations with maximum binding energy. The increase in nucleation density for etched GaN layers also result in dense nanorods which exhibit better excitonic emission. Our studies suggest that ZnO nanostructures with improved optical and structural properties can be grown on etched-GaN as well as AlGaN/GaN heterostructures as the bottom layer. It is interesting to observe that the bottom GaN layer can be easily employed to determine the optical quality of ZnO layer.
Introduction
GaN and ZnO are well known wide band gap semiconductors with high excitonic energy and low power thresholds at room temperature.1,2 Unlike p-type ZnO, n-type ZnO and n/p-type GaN can be grown with good structural and electrical characteristics and the results are reproducible. The reason why researchers worldwide have focused their attention to the growth of various types of heterostructures involving GaN and ZnO is the challenges in making p-type ZnO. Growth of one dimensional nanostructures of either ZnO or GaN on a thin film of the other has also gained much importance because such structures exhibit photonic and electronic confinement in two dimensions with potential nanoscale optoelectronic devices. Nanostructures of ZnO have high optical gain and low threshold stimulated emission required in lasers,3 which is one of the main reasons we focus our efforts on the growth of ZnO nanostructures on GaN. It is possible to grow ZnO films on a GaN template using molecular beam epitaxy,4 metal–organic chemical vapor deposition,5 magnetron sputtering6 as well as pulsed laser deposition (PLD).7 Columnar growth of any one of the semiconductor layer on the other in GaN/ZnO based heterostructures can provide an effective route to relax stress and improve the quality of the epitaxial layers. The formation of columnar nanostructure allows the combination of materials with large lattice mismatch simultaneously providing us with interesting physical properties. Different morphology of the bottom III–V nitride layer has been utilized to grow optimum quality ZnO nanostructures. In most of the Vapour Liquid Solid (VLS) growth techniques for nanostructures it has been found that a metal catalyst is used and is organized spatially over the growth layer.8 This mechanism enables a controlled growth of nanostructures.In all the above mentioned cases the catalyst particle initiates the growth of dislocation-driven nanowires/nanotubes. However, in the present work, we demonstrate the growth of ZnO nanostructures on nitride-layer, initiated intentionally and driven by dislocations. This is achieved by making use of the dislocations that are contained within the GaN thin films. Growth of ZnO nanostructures on GaN based heterostructures is essential for optical biosensing studies.9 For this reason AlGaN/GaN heterostructure was also chosen as one of the bottom layers for depositing ZnO nanostructures.
Experimental details
GaN based epitaxial layers and heterostructures were deposited on c-sapphire substrates by metal organic chemical vapour deposition (MOCVD), Aixtron 200/4 RF-S system. Trimethyl gallium (TMG), trimethyl aluminum (TMA) and ammonia (NH3) where used as Ga, Al and N sources to grow GaN (sample A), etched GaN (sample B) and AlGaN/GaN heterostructure (sample C). One of the GaN film was etched using H3PO4 (85%) acid. These layers were then used as templates for growing ZnO nanorods by a hydrothermal solution technique. We then followed a two-step hydrothermal growth process10 to obtain high-density, aligned ZnO nanorod arrays over the GaN, etched GaN and AlGaN/GaN heterostructure. Reciprocal space mapping (RSMs) of as grown layers was investigated using HRXRD [PAN analytical MRD PW 3050/65]. A reciprocal space map is a two dimensional measurement that combines 2θ scans and ω-scans of HRXRD measurements and it can help us to determine deformation of the ZnO layer on GaN, etched GaN and AlGaN/GaN layers. Powder XRD measurements [PAN analytical X'Pert Powder XRD system] were used to analyze the structure of ZnO nanostructure grown on GaN based heterostructures. Morphology of the as grown GaN layers and etched GaN layers and after the growth of ZnO nanostructures was investigated using scanning electron microscopy (SEM). The optical properties of the nanostructure were examined using photoluminescence spectrometer at room temperature with a power of 30 mW and laser spot size of 30 μm (Spectra Physics, wavelength-tunable argon ion laser, λexcitation = 244 nm). The crystallinity and structure of the ZnO nanostructure grown on GaN based heterostructure was studied using a confocal Raman microscope (Alpha 300, WITec).Results and discussion
Structural investigation of GaN thin films and heterostructure grown on sapphire was carried out using HRXRD. The X-ray Rocking Curve (XRC) analysis of GaN was carried out for sample A, B and C as shown in Fig. 1(a–c). The full width half maximum (FWHM) of sample A, sample B and sample C was found to be 457 arcsec, 525 arcsec and 382 arcsec respectively. It is clear that post-etching, the FWHM of GaN surface broadens due to the presence of etched defects. The etched layer has the highest FWHM due to the presence of more dislocations in the form of hexagonal pits (Fig. 4). The lower FWHM value for AlGaN/GaN heterostructures can be attributed to the presence of a finite number of buffer layers during initial growth. In Fig. 1(d) we present the ω–2θ scans of AlGaN/GaN heterostructure. The Al mole fraction in the AlGaN layer was found to be 21%. Structural characterization reveals the crystalline nature of nitride layers which shows that these substrates are suitable for the growth of nanostructures. | ||
| Fig. 1 X-Ray Rocking Curve (XRC) of GaN layers (before depositing ZnO nanostructures) in sample A, sample B and sample C (d) ω–2θ scan of AlGaN/GaN heterostructure. | ||
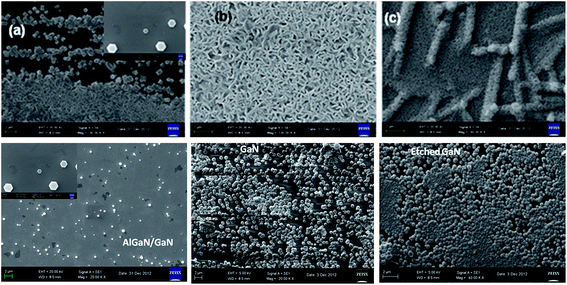
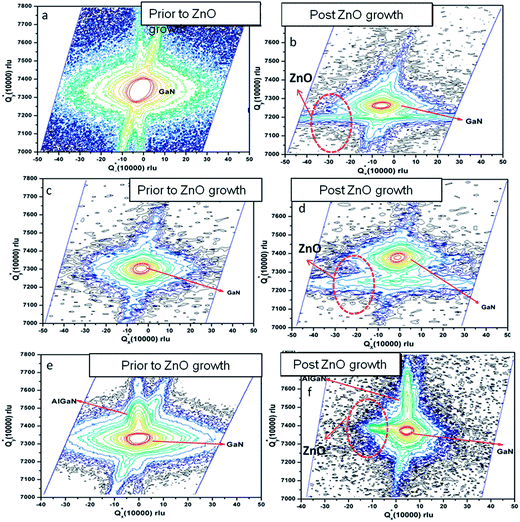
The impact of strain upon ZnO layer grown on GaN layers and AlGaN/GaN heterostructures was investigated by HRXRD (0002) reciprocal-space mapping (RSMs). Fig. 2 shows the RSMs on sample A, B and C. It is clear that ZnO peak overlaps with the GaN peak. Also variation in peak intensity before and after the growth of ZnO nanostructure on GaN based heterostructures is observed. There is no indication of layer-relaxation because all counts are detected at a Qx values equal to that of the GaN peak and the position of the reciprocal lattice point corresponding to ZnO layer with respect to GaN shows presence of strain in that layer.11 ZnO nanostructures were grown on these crystalline layers using a hydrothermal technique.10 Fig. 3 shows the X-ray diffraction pattern of ZnO nanostructures grown on nitride layers and the peak positions indicate the reflections of wurtzite ZnO nanostructures,12 while the asterisk indicates the reflection corresponding to aluminium sample holder. The diffraction peaks have been identified in the figure legend. Inset reveals the overlapped peaks of the spectra. Microstructural analysis of samples A, B and C after the growth of ZnO nanostructures shows a different morphology in each case. It is well known that GaN thin films grown on sapphire substrates contain high densities of dislocations.13 These dislocations are exposed on the surface and are the ones which help to propagate the growth of ZnO nanotubes/nanorods directly from the aqueous solutions. The growth is favourable also because of the fact that ZnO and GaN have the same wurtzite structure with matched basal planes of 3.249 Å and 3.186 Å respectively.14 Here, in the case of etched GaN (sample B), a dense and packed growth of ZnO nanorods takes place, whereas for unetched GaN sample (sample A) the growth is mostly in the form of nanotubes (Fig. 4a and b). A prolonged exposure to the growth solution also helps in formation of secondary nucleation sites (Fig. 4c and d). These randomly distributed nucleation sites leads to the formation of nanoflower like structures. The nature of ZnO nanostructures grown on AlGaN/GaN layer (sample C) is completely different. Initial growth features indicate vertical growth of ZnO rods (Fig. 5(a)) well separated from each other. This is slowly and continuously followed by dense growth, where the rods tend to push each other in a bid to outgrow, ultimately leading to a flake like structure (Fig. 5(b)). Prolonged growth time leads to the second nucleation layer. The second nucleation layer grows only along certain specific directions, preferably were the defect density seems to favor the nucleation. This leads to the formation of unique criss-crossed structures on the first growth layer (Fig. 5(c)). SEM images of partial growth (Fig. 5) for same deposition time (5 min) on all samples prove beyond doubt that the nucleation density is highest on the etched GaN. We also calculate the dislocation density [DB] for the samples from the (002) peak of GaN using the following formula for random dislocation distribution proposed by Dunn and Kogh15
 | ||
| Fig. 2 RSM studies of (a) GaN, (b) ZnO on GaN, (c) etched-GaN, (d) ZnO on etched GaN, (e) AlGaN/GaN and (f) ZnO on AlGaN/GaN layers. | ||
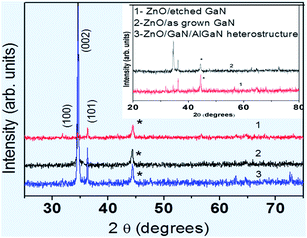 | ||
| Fig. 3 XRD pattern of samples obtained after the growth of ZnO nanostructures. All the peaks correspond to ZnO. Inset reveals the overlapped peaks of the spectra. | ||
 | ||
| Fig. 4 SEM images (top view) – ZnO nanorods grown on (a) etched-GaN (b) GaN. Images (c) and (d) show the second layer of nucleation with flower like structures and different morphologies. | ||
The dislocation density for as grown GaN is found to be 4.1 × 108 cm−2, that for etched GaN it is 5.6 × 108 cm−2 and for AlGaN/GaN it is ∼2.9 × 108 cm−2. The etch pit density of etched GaN is found to be 6.2 × 108 cm−2 using the surface to volume ratio of the concerned sample.
Upon correlating the SEM images of partial growth in Fig. 5 it is clear that the nucleation density on the bottom AlGaN/GaN, as grown GaN and etched GaN layers increase with dislocation density. The etch pit density of etched GaN definitely enhances the nucleation density from the beginning. For AlGaN/GaN bottom layer, the ZnO rods are initially well separated perfect standing hexagons, which collide into one another giving rise to flake like structures as the deposition time increases.
In Fig. 6(bottom) the images (a) and (b) correspond to the cross-section of the AlGaN/GaN and GaN layer for samples prior to the growth of ZnO layer. Cross-sectional images also show vertical ZnO rods on etched GaN layer at lower magnification (c) and higher magnification (d). The inset in (d) corresponds to ZnO layer grown on AlGaN/GaN layer [Fig. 5(b)].
It is interesting to observe here that small variations in the morphology/nature of bottom layer can control the nucleation rate and growth of top layer in a heterostructure. To explain the above growth mechanism we first take the case of etched GaN (schematic shown in Fig. 6(bottom)). The presence of etched pits provides an enhanced surface area for nucleation to take place. If we refer to the theory of nucleation, growth species prefer to condense on locations with maximum binding energy E,16,17
When the GaN films and heterostructures are subjected to growth solutions of ZnO with low-supersaturation, the dislocations present act as “seed” and help in propagating the growth of ZnO generating nanorods/nanotubes arrays of ZnO from the growth solution. Etching the GaN film gives way to small etch pits at the core of many dislocations with pure or partial screw character.19 As shown in the schematic (Fig. 6) the nucleation happens in various steps and some points at the etched film are more favorable as nucleation sites than others. At first the pits are filled with ZnO nuclei, followed by nucleation on the film surface. As a result of this growth pattern dense ZnO rods can be grown on the etched-GaN layer (sample B). The ZnO nanorod growth densities will be similar to the dislocation density observed for such GaN-substrates, suggesting that the strain energy created by dislocations in these pits is released with the formation of the nanorods. The final morphology however, depends on the properties of the material and the nature of dislocation. When similar growth process is used for as grown GaN films (sample A), the nanorods are replaced by nanotubes, the density of tubes being less compared to those of etched GaN films. This can be attributed to the fact that the absence of etched pits minimizes the nucleation process, as a result of which the nucleation density decreases.
Photoluminescence (PL) studies were performed prior to ZnO growth on the GaN layers to investigate the optical properties of bottom layer (Fig. 8). The as grown GaN (sample A) emits a excitonic GaN near band edge emission at about 361 nm. For sample B and C, the excitonic emission appears to be red shifted giving a broader emission in presence of etched pits in sample B and AlGaN/GaN heterostructures in sample C respectively. In sample C we can also observe the peak corresponding to AlGaN emission around 329 nm. PL studies were performed again after the growth of ZnO nanostructures on each of these layers (Fig. 9). The relative intensity of emission around 362 nm to emission around 370 nm decreases after ZnO nanostructure growth clearly indicating the contribution to PL from ZnO layer. For sample A, the peak at 362.5 nm is due to transitions involving bound exciton (GaN) and that at 371.5 nm is due to the excitonic emissions from the core ZnO nanotube. This characteristic emission at 371.5 nm has been observed from ZnO–GaN coaxial heterostructure at a low temperature of 80 K,20 whereas in our case we observe it at room temperature. For samples B and C, the near-band-edge (NBE) emissions from GaN layers occur at 361.7 nm and that from ZnO occur at 370 nm. For sample C we also observe a corresponding AlGaN peak emission around 329 nm. This difference in the nature of PL emission can be explained to some extent by the nature of defects in nanorods and nanotubes grown. In the case of sample B and C the ZnO nanorods are solid and are responsible for higher ratio of excitonic to visible emission. In sample A the lower ratio of excitonic to defect emission is attributed to the hollow structure of ZnO nanotube. ZnO nanotubes offer a larger circumference when compared to a solid ZnO core nanorod in the heterostructures.20 Hence, the UV to defect emission is much higher in case of ZnO nanorods grown on etched-GaN layer (sample B). The visible band located at 400–500 nm is known to come from the deep-level emission caused by oxygen vacancies, including 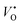 and
and 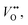 where
where 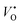 is the oxygen vacancy at 2.0 eV below the conduction band, whereas
is the oxygen vacancy at 2.0 eV below the conduction band, whereas 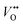 is the oxygen vacancy at 2.2 eV below the conduction band.21–24 The emission around 400 nm can also result from the presence of zinc interstitials. As the depletion region at the ZnO–GaN surface contains
is the oxygen vacancy at 2.2 eV below the conduction band.21–24 The emission around 400 nm can also result from the presence of zinc interstitials. As the depletion region at the ZnO–GaN surface contains 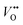 rather than
rather than 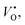 this implies that the ZnO nanotubes having a larger surface area would have a large amount of
this implies that the ZnO nanotubes having a larger surface area would have a large amount of 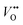 . On the contrary, the ZnO nanorods would have a higher concentration of
. On the contrary, the ZnO nanorods would have a higher concentration of 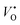 instead of
instead of  . Therefore, the PL results indicate that the ZnO nanotubes would prefer to perform blue/green luminescence, whereas nanostructures grown on etched-GaN and AlGaN/GaN layers (sample B and C) would radiate green/yellow lights. Due to lower surface area of contact at the heterostructure interface, the ZnO nanorods exhibit better PL properties as compared to the ZnO nanotubes in terms of the excitonic emission as well as the ratio of excitation to visible emission.
. Therefore, the PL results indicate that the ZnO nanotubes would prefer to perform blue/green luminescence, whereas nanostructures grown on etched-GaN and AlGaN/GaN layers (sample B and C) would radiate green/yellow lights. Due to lower surface area of contact at the heterostructure interface, the ZnO nanorods exhibit better PL properties as compared to the ZnO nanotubes in terms of the excitonic emission as well as the ratio of excitation to visible emission.
 | ||
| Fig. 8 PL spectra of as grown GaN, etched-GaN and AlGaN/GaN heterostructure prior to the growth of ZnO nanostructures. | ||
Characterization of samples by Raman spectroscopy (Fig. 10) gives us further information about the quality of samples. The weak Raman peak, found in all the samples, at 330 cm−1 corresponds to that of ZnO. The relative ratio of intensity of ZnO nonpolar optical phonon E2 mode (437 cm−1) to the intensity of 330 cm−1 is highest for the ZnO layer grown on etched-GaN. The mode appearing at 569 cm−1 (sample A) and 575 cm−1 (sample B and C) corresponds to the GaN-E2 phonon mode. The A1 LO-GaN peak (733 cm−1) appears only in case of sample A. In the samples where the A1 LO line is absent, the FWHM of the GaN-E2 peak is much wider and weak. GaN-E2 mode being sensitive to the internal stress and residual strains in the layer, this broadening suggests that residual stress and strain is higher for etched-GaN (sample B) and AlGaN/GaN layer (sample C) and is at least partly responsible for broadening and quenching of the A1 LO line. GaN-E2 shifts very little from the bulk unstrained value ∼568 cm−1 to 569 cm−1 for unetched-GaN layer (sample A), whereas the strain in the bottom etched-GaN (sample B) and ALGaN/GaN (sample C) layer leads to a shift by 7 cm−1 to 575 cm−1.25–29
 | ||
| Fig. 10 Raman spectra of ZnO (a) nanotubes (b) nanorods and nanoflakes grown on GaN, etched-GaN and AlGaN/GaN layers respectively. | ||
Conclusion
ZnO nanostructures/GaN based heterostructures have been fabricated using hydrothermal and MOCVD techniques. These heterostructures have been investigated using HRXRD, SEM, PL and Raman spectroscopy. The present study shows that ZnO nanostructures with good optical and structural properties can be grown on etched-GaN as well as AlGaN/GaN heterostructures as compared to as grown GaN layers. Etched-GaN layers provide a modified and enlarged surface area for growth of ZnO nanorods. The increase in nucleation density for etched GaN layers also results in dense nanorods which exhibit low defect emission. For ZnO nanostructures grown on a AlGaN/GaN layer the density of nanorods is so high that they push each other in the process to form a unique microstructure. These layers also show a low defect emission as compared to those grown directly on GaN films. It is interesting to observe that the bottom GaN layer can be easily employed to determine the morphology as well as optical properties of ZnO nanostructured layer in a ZnO–GaN heterostructure. This study can be further extended in the future to investigate the electrical properties of ZnO–GaN heterostructures.Acknowledgements
Shubra Singh would like to acknowledge the DST-INSPIRE Faculty fellowship 2012 [IFA-PH-08] for their support.References
- S.-K. Hong, H.-J. Ko, Y. Chen and T. Yao, J. Cryst. Growth, 1992, 117, 366 CrossRef.
- A. Urbieta, P. Fernández, J. Piqueras, Ch. Hardalov and T. Sekiguch, J. Phys. D: Appl. Phys., 2001, 34, 2945 CrossRef CAS.
- Y. Chen, D. M. Baghall, H. Koh, K. Park, K. Hiraga, Z. Zhu and T. Yao, J. Appl. Phys., 1998, 84, 3912 CrossRef CAS PubMed.
- S. Hong, T. Hanada, H. Ko, Y. Chen and T. Yao, Phys. Rev. B: Condens. Matter Mater. Phys., 2002, 65, 115331 CrossRef.
- T. Ben-Yaacov, T. Ive, C. G. Van de Walle, U. K. Mishra, J. S. Speck and S. P. Denbaars, J. Electron. Mater., 2010, 39, 608–611 CrossRef CAS PubMed.
- J. N. Dai, X. Y. Han, Z. H. Wu, C. H. Yu, R. F. Xiang, Q. H. He, Y. H. Gao, C. Q. Chen, X. H. Xiao and T. C. Peng, J. Alloys Compd., 2010, 489(2), 519–522 CrossRef CAS PubMed.
- X. Han, Y. Gao, J. Dai, C. Yu, Z. Wu, C. Chen and G. Fang, J. Phys. D: Appl. Phys., 2010, 43, 145102 CrossRef.
- I. Levin, A. Davydov, B. Nikoobakht, N. Sanford and P. Mogilevsky, Appl. Phys. Lett., 2005, 87, 103110 CrossRef PubMed.
- H.-Y. Shih, Y.-T. Chen, C.-M. Wei, M.-H. Chan, J.-K. Lian, Y.-F. Chen and T.-Y. Lin, J. Phys. Chem. C, 2011, 115, 14664–14667 CAS.
- M. Guo, P. Diao and S. Cai, J. Solid State Chem., 2005, 178, 1864–1873 CrossRef CAS PubMed.
- E. Przezdziecka, A. Wierzbicka, A. Reszka, K. Goscinski, A. Droba, R. Jakiela, D. Dobosz, T. A. Krajewski, K. Kopalko, J. M. Sajkowski, M. Stachowicz, M. A. Pietrzyk and A. Kozanecki, J. Phys. D: Appl. Phys., 2013, 46, 035101 CrossRef.
- S. Singh, E. Senthil Kumar and M. S. Ramachandra Rao, J. Nanosci. Nanotechnol., 2009, 9, 1–5 CrossRef PubMed.
- S. Y. Huang and J. R. Yang, Jpn. J. Appl. Phys., 2008, 47, 7998–8002 CrossRef CAS.
- R. D. Vispute, V. Talyansky, S. Choopun, R. P. Sharma, T. Venkatesan, M. He, X. Tang, J. B. Halpern, M. G. Spencer, Y. X. Li, L. G. Salamanca-Riba, A. A. Iliadis and K. A. Jones, Appl. Phys. Lett., 1998, 73, 348–350 CrossRef CAS PubMed.
- C. G. Dunn and E. F. Kogh, Acta Metall., 1957, 5, 548 CrossRef CAS.
- Laboratory Manual on Crystal Growth, ed. I. Tarjan and M. Matrai, AkadÈmiai KiadÛ, Budapest, 1972, pp. 29–30 Search PubMed.
- A. Prasad, S. Mensah, J. Wang, A. Pandey and Y. K. Yap, Mater. Res. Soc. Symp. Proc, 2008, vol. 1057, Materials Research Society, 1057-II13-02 Search PubMed.
- R. Dumpala, B. Ramamoorthy and M. S. Ramachandra Rao, Appl. Surf. Sci., 2014, 545–550 CrossRef CAS PubMed.
- S. A. Morin and S. Jin, Nano Lett., 2010, 10, 3459–3463 CrossRef CAS PubMed.
- Y. J. Hong, J.-M. Jeon, M. Kim, S.-R. Jeon, K. H. Park and G.-C. Yi, New J. Phys., 2009, 11, 125021 CrossRef.
- Y. Y. Peng, T. E. Hsieh and C. H. Hsu, Nanotechnology, 2006, 17, 174 CrossRef CAS.
- C. C. Wu, D. S. Wuu, P. R. Lin, T. N. Chen and R. H. Horng, Cryst. Growth Des., 2009, 9, 4555 CAS.
- K. Vanheusden, W. L. Warren, C. H. Seager, D. R. Tallant, J. A. Voigt and B. E. Gnade, J. Appl. Phys., 1996, 79, 7983 CrossRef CAS PubMed.
- S. A. M. Lima, F. A. Sigoli, M. Jafelicci, Jr and M. R. Davolos, Int. J. Inorg. Mater., 2001, 3, 749 CrossRef CAS.
- G. Popovici, G. Y. Xu, A. Botchkarev, W. Kim, H. Tang, A. Salvador, H. Morkoc, R. Strange and J. O. White, J. Appl. Phys., 1997, 82, 4020 CrossRef CAS PubMed.
- D. Kirillov, H. Lee and J. S. Harris, Jr, J. Appl. Phys., 1996, 80, 4058 CrossRef CAS PubMed.
- C. Kisielowski, J. Kruger, S. Ruvimov, T. Suski, J. W. Ager III, E. Jones, Z. Liliental-Weber, M. Rubin, E. R. Weber, M. D. Bremer and R. F. Davis, Phys. Rev. B: Condens. Matter Mater. Phys., 1996, 54, 17745 CrossRef CAS.
- T. Kozawa, T. Kachi, H. Kano, Y. Taga, M. Hashimoto, N. Koide and K. Manabe, J. Appl. Phys., 1994, 75, 1096–1098 CrossRef PubMed.
- P. Perlin, C. J. Carillon, J. P. Itie, A. S. Miguel, I. Grezgory and A. Polian, Phys. Rev. B: Condens. Matter Mater. Phys., 1992, 45, 83 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2014 |