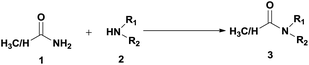
A binuclear Mn(II) complex as an efficient catalyst for transamidation of carboxamides with amines†
Divya Pratap
Singh
,
Bharat Kumar
Allam
,
Krishna Nand
Singh
and
Vinod Prasad
Singh
*
Department of Chemistry, Faculty of Science, Banaras Hindu University, Varanasi 221005, India. E-mail: singvp@yahoo.co.in; Tel: +91 9450145060
First published on 12th November 2013
Abstract
A binuclear Mn(II) complex has been synthesized and characterized by different structural methods. The complex contains two unique oxo-bridged metal centres and has been explored as an excellent catalyst for transamidation of carboxamides with amines under solvent-free conditions.
Schiff bases are privileged ligands with important applications.1 The redox rich chemistry of manganese centres is usually related to their function and applications in metalloenzymes, catalysis, imaging, neurotoxicity, and magnetic materials.2 The Mn(II) ions also play a key role in the development of medical diagnostic tools of lower toxicity. High-spin Mn(II) materials can efficiently enhance the relaxation rates of water protons and thus act as contrast agents for magnetic resonance imaging (MRI).2c
The synthesis and catalytic applications of manganese(II) binuclear complexes have been developed as an important research area.3 It is well-known that electronic communication between manganese centres within a binuclear complex affects their redox properties. The existence of a labile coordination site is an important prerequisite for the involvement of an inner-sphere electron transfer mechanism in the activation of small molecules by the metal centres. It has been investigated that the binuclear Schiff base complexes of transition metal ions have the potential of catalyzing reactions more effectively with different chemo-, regio- or stereo-selectivity than those of corresponding mononuclear ones due to the synergic effect of two metal ions.4–7
Transamidation is a valuable tool for the preparation of bio-inspired materials and represents one of the most convenient and straightforward method for exchanging the constituents of two different amide groups.8 To accomplish an efficient transamidation under mild conditions, aluminium, copper, cerium, zirconium, hydroxylamine hydrochloride, ammonium bromide, L-proline and boric acid catalyzed methods have been recently developed by different research groups.9–12 Hypervalent iodine catalyzed13 and 1,4-dioxane mediated14 transamidation protocols have also been explored by our group. In order to further advance these studies, we chose to investigate the catalytic use of a newly synthesized binuclear manganese(II) complex for this process. Recently, manganese based catalysts have emerged as effective promoters for various organic transformations15 and bio-chemical reactions,16 but their use for a demanding transamidation reaction has not been explored so far. Establishing a practical synthetic protocol for transamidation using manganese based catalysts would thus be highly desirable.
We report herein the synthesis of a binuclear manganese(II) complex, [Mn(Hbpoh)(OAc)(H2O)]2·6H2O, where, H2bpoh = (N′1E,N′2E)-N′1,N′2-bis(phenyl (pyridin-2-yl)methylene)oxalo-hydrazide, which has been characterized by spectroscopic and single crystal X-ray methods. An investigation on the catalytic role of the complex towards the transamidation of carboxamides with amines has also been undertaken under solvent-free conditions.
Single crystal X-ray structure of the complex (Fig. 1) shows that out of the two ![[double bond splayed left]](https://www.rsc.org/images/entities/char_e009.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O groups present in ligand, one bonds with Mn(II) in the form of enolate ion and the other
O groups present in ligand, one bonds with Mn(II) in the form of enolate ion and the other ![[double bond splayed left]](https://www.rsc.org/images/entities/char_e009.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O remains free. The Mn–O–Mn bridged complex has two octahedral Mn centres, each coordinated with the ligand through azomethine–nitrogen, pyridine–nitrogen and a carbonylate–oxygen, and form two five membered chelate rings. In addition, one water molecule and one acetate ion occupy the remaining octahedral coordination sites of each metal centre. The average bond lengths of the complex are: O(1)–C(13) = 1.260(4), N(3)–N(2) = 1.393(5), N(2)–C(6) = 1.286(5), N(3)–C(13) = 1.310(5), N(1)–C(1) = 1.340(6), C(5)–N(1) = 1.351(4), C(5)–C(6) = 1.480(6), C(6)–C(7) = 1.496(4) Å, which are longer or shorter than those of the corresponding distances in ligand and suggest considerable delocalization of charge.
O remains free. The Mn–O–Mn bridged complex has two octahedral Mn centres, each coordinated with the ligand through azomethine–nitrogen, pyridine–nitrogen and a carbonylate–oxygen, and form two five membered chelate rings. In addition, one water molecule and one acetate ion occupy the remaining octahedral coordination sites of each metal centre. The average bond lengths of the complex are: O(1)–C(13) = 1.260(4), N(3)–N(2) = 1.393(5), N(2)–C(6) = 1.286(5), N(3)–C(13) = 1.310(5), N(1)–C(1) = 1.340(6), C(5)–N(1) = 1.351(4), C(5)–C(6) = 1.480(6), C(6)–C(7) = 1.496(4) Å, which are longer or shorter than those of the corresponding distances in ligand and suggest considerable delocalization of charge.
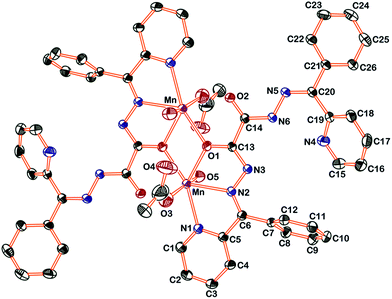 | ||
| Fig. 1 ORTEP diagram of [Mn(Hbpoh)(OAc)(H2O)]2·6H2O showing atomic numbering scheme with ellipsoid of 30% probability (hydrogen atoms and solvent cage are omitted for clarity). | ||
The Mn–O(1), Mn–N(1) and Mn–N(2) bond distances are 2.209(4) Å, 2.307(4) Å and 2.323(2) Å, respectively, which fall in the range reported for Mn(II) distorted octahedral complexes.17 The cis-angles in the basal plane involving the bridged carbonylate–oxygen and azomethine–nitrogen (O(1)–Mn(1)–N(2) = 66.92(7)°), (N(2)–Mn (1)–N(1) = 69.19(7)°) show deviation from the ideal value of 90° suggesting that the octahedral geometry is slightly distorted due to chelation effect.18 The C(6)–N(2) distance (1.286(5) Å) in the complex is longer than the ligand (1.294(5) Å) due to coordination of azomethine–nitrogen to the metal ion. The N(3)–C(13) distance (1.310(5) Å) in the complex is significantly shorter than the free ligand (1.352(5) Å) as a result of deprotonation of immine group and formation of ![[double bond splayed left]](https://www.rsc.org/images/entities/char_e009.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N. The O(1)–C(13) bond length (1.260(4) Å) is significantly longer than the free ligand (1.208(5) Å) due to enolization of
N. The O(1)–C(13) bond length (1.260(4) Å) is significantly longer than the free ligand (1.208(5) Å) due to enolization of ![[double bond splayed left]](https://www.rsc.org/images/entities/char_e009.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O group during complexation. These evidences suggest that the π electrons originated from deprotonation of the oxamido group, are delocalized to form a conjugated system including N(3), C(13) and O(1). The torsion angles N(1)–C(5)–C(6)–C(7) [172.6(4)°], O(1)–C(13)–N(3)–N(2) [–1.4(6)°], N(1)–C(5)–C(6)–N(2) [–8.1(6)°] and N(3)–N(2)–C(6)–C(7) [–2.5(6)°] indicate that O(1)–N(2), N(1)–N(2) and N(3)–C(7) are syn-periplanar to each other but N(1)–C(7) is anti-periplanar to each other.
O group during complexation. These evidences suggest that the π electrons originated from deprotonation of the oxamido group, are delocalized to form a conjugated system including N(3), C(13) and O(1). The torsion angles N(1)–C(5)–C(6)–C(7) [172.6(4)°], O(1)–C(13)–N(3)–N(2) [–1.4(6)°], N(1)–C(5)–C(6)–N(2) [–8.1(6)°] and N(3)–N(2)–C(6)–C(7) [–2.5(6)°] indicate that O(1)–N(2), N(1)–N(2) and N(3)–C(7) are syn-periplanar to each other but N(1)–C(7) is anti-periplanar to each other.
The molecular structure of the complex is stabilized by intra-molecular C–H⋯π interactions (Fig. 2), and different intra- and inter-molecular hydrogen bonding interactions between water molecules and ![[double bond splayed left]](https://www.rsc.org/images/entities/char_e009.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O of ligand (Fig. S4†). The pyridine ring proton approaches the centroid of the phenyl ring resulting in C–H⋯π interaction with a contact distance of 3.034 Å,19 while phenyl ring proton approaches the centroid of the pyridine ring resulting in C–H⋯π interaction with a contact distance of 3.202 Å.
O of ligand (Fig. S4†). The pyridine ring proton approaches the centroid of the phenyl ring resulting in C–H⋯π interaction with a contact distance of 3.034 Å,19 while phenyl ring proton approaches the centroid of the pyridine ring resulting in C–H⋯π interaction with a contact distance of 3.202 Å.
The IR spectral bands observed at 3387, 1690 and 1582 cm−1 in the free ligand are assigned to ν(N–H), ν(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) and ν(C
O) and ν(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), respectively.20 The ν(N–H), ν(C
N), respectively.20 The ν(N–H), ν(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), and ν(C
N), and ν(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) bands in the metal complex appear at the same position as in the ligand, while new ν(C
O) bands in the metal complex appear at the same position as in the ligand, while new ν(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N) and ν(C–O) bands appear at 1547 and 1250 cm−1 as a result of enolization of one carbonyl group during complex formation. In the complex, one of the ν(C
N) and ν(C–O) bands appear at 1547 and 1250 cm−1 as a result of enolization of one carbonyl group during complex formation. In the complex, one of the ν(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N) shifts towards lower wave number,5 indicating coordination with azomethine–nitrogen.
N) shifts towards lower wave number,5 indicating coordination with azomethine–nitrogen.
The complex shows μeff value 5.95 B.M. corresponding to five unpaired electrons. Electronic spectra of the complex exhibits two d–d bands of very weak intensity at 620 and 495 nm which may be assigned to 6A1g → 4T1g(G) and 6A1g → 4T2g(G) suggesting an octahedral environment around the metal ion.21
To investigate the catalytic efficiency of the complex, a model transamidation reaction between acetamide and aniline to afford product 3j was conducted by varying different conditions (Table 1).
| Entry | Catalyst mol (%) | Solvent | T (°C) | Yield 3jb (%) |
|---|---|---|---|---|
| a Using acetamide (10 mmol) and aniline (10 mmol) for 24 h. b Isolated yield. | ||||
| 1 | Complex (5) | — | 100 | 70 |
| 2 | Complex (5) | — | 120 | 82 |
| 3 | Complex (5) | — | 130 | 80 |
| 4 | Complex (5) | Toluene | 120 | 40 |
| 5 | Complex (5) | t AmOH | 120 | 55 |
| 6 | Complex (5) | DMSO | 120 | 37 |
| 7 | Complex (3) | — | 120 | 75 |
| 8 | Complex (10) | — | 120 | 82 |
| 9 | MnCl2·4H2O (5) | — | 120 | 30 |
| 10 | Mn(OAc)2·4H2O (5) | — | 120 | 40 |
| 11 | MnO2 (5) | — | 120 | 35 |
| 12 | Mn(NO3)2·4H2O (5) | — | 120 | 30 |
| 13 | H2bpoh (10) | — | 120 | n.r |
| 14 | — | — | 120 | n.r |
The addition of complex was assumed to facilitate the transamidation reaction through the coordination and activation of amide oxygen. Initially 5 mol% of the complex was screened at 100 °C under solvent-free conditions affording 70% product yield after 24 h (entry 1). When the temperature was increased to 120 °C using the same conditions, a commendable increase in the yield (82%) was noticed (entry 2). The reaction was then carried out using different solvents viz. toluene, tAmOH and DMSO, but all of them ever provided a much lower yield (entries 4–6). A lower catalyst loading led to a lower yield, whereas a high catalyst loading did not improve the yield (entries 7 and 8). The reaction failed to proceed in the absence of complex, thus necessitating the use of the catalyst (entry 14). The ligand H2bpoh (10 mol%) alone was also found to be ineffective (entry 13). Various Mn(II) salts such as MnCl2·4H2O, Mn(OAc)2·4H2O, MnO2 and Mn(NO3)2·4H2O were also tested as catalysts but the reactions invariably ended with low product yields (30–40%, entries 9–12). To extend the scope and versatility of the present findings, different carboxamides were allowed to react with diverse aliphatic and aromatic amines under the optimized set of reaction conditions to deliver the corresponding products 3a–3r efficiently in good to excellent yields (50–89%, Table 2).
The transamidation reactions of formamide and acetamide underwent smoothly with different amines namely morpholine, 4-chloroaniline, 3-hydroxyaniline, 2-aminopyridine, N-methylaniline, 3-methylaniline, 4-hydroxyaniline, 4-methoxyaniline, benzylamine, 2-metoxyaniline and phenylhydrazine to deliver the corresponding formylated and acetylated amines (Table 2). Aromatic primary amines substituted with electron-donating groups at para-position enhanced the product yield considerably (3h & 3l). Halogen substituted aromatic amines also participated well in the reactions (3b & 3p). A typical heterocyclic amine, 2-aminopyridine underwent the transamidation efficiently to deliver the N-(pyridin-2-yl)formamide in 79% yield (3d). As regards the steric effects, aromatic amine having ortho substituent gave rise to lower product yield (3m). Dicyclohexylamine did not react at all. A little decrease in the yield was noticed for hydroxyl substituted aromatic amines (3c, 3g & 3o).
Conclusions
The synthesis and characterization of a binuclear Mn(II) complex have been undertaken, and the catalytic efficacy of the synthesized complex has been successfully explored for the transamidation reaction.Acknowledgements
The authors D. P. S. and B. K. A. thank Council of Scientific and Industrial Research (CSIR), New Delhi for financial assistance in the form of Senior Research Fellowship.Notes and references
- (a) P. W. Roesky, A. Bhunia, Y. Lan, A. K. Powell and V. Kureti, Chem. Commun., 2011, 47, 2035–2037 RSC; (b) A. Decortes, A. M. Castilla and A. W. Kleij, Angew. Chem., Int. Ed., 2010, 49, 9822–9837 CrossRef CAS PubMed; (c) S. Guieu, A. K. Crane and M. J. MacLachlan, Chem. Commun., 2011, 47, 1169–1171 RSC.
- (a) K. J. Kim, M. S. Park, J. H. Kim, U. Hwang, N. J. Lee, G. Jeong and Y. J. Kim, Chem. Commun., 2012, 48, 5455–5457 RSC; (b) J. D. Aguirre and V. C. Culotta, J. Biol. Chem., 2012, 287, 13541–13548 CrossRef CAS PubMed; (c) D. Pan, S. D. Caruthers, A. Senpan, A. H. Schmieder, S. A. Wickline and M. L. Gregory, Wiley Interdiscip. Rev.: Nanomed. Nanobiotechnol., 2011, 3, 162–173 CrossRef CAS PubMed.
- (a) J. W. de Boer, W. R. Browne, S. R. Harutyunyan, L. Bini, T. D. Tiemersma–Wegman, P. L. Alsters, R. Hage and B. L. Feringa, Chem. Commun., 2008, 3747–3749 RSC; (b) G. K. Y. Ng, J. W. Ziller and A. S. Borovik, Chem. Commun., 2012, 48, 2546–2548 RSC; (c) M. K. Coggins, X. Sun, Y. Kwak, E. I. Solomon, E. Rybak-Akimova and J. A. Kovacs, J. Am. Chem. Soc., 2013, 135, 5631–5640 CrossRef CAS PubMed.
- J. Y. Lee, O. K. Farha, J. Roberts, K. A. Scheidt, S. T. Nguyen and J. T. Hupp, Chem. Soc. Rev., 2009, 38, 1450–1459 RSC.
- D. P. Singh, D. S. Raghuvanshi, K. N. Singh and V. P. Singh, J. Mol. Catal. A: Chem., 2013, 379, 21–29 CrossRef CAS PubMed.
- G. González-Riopedre, M. I. Fernández-Garcia, A. M. González-Noya, M. Á. Vázquez-Fernández, M. R. Bermejo and M. Maneiro, Phys. Chem. Chem. Phys., 2011, 13, 18069–18077 RSC.
- D. Balcells, P. Moles, J. D. Blakemore, C. Raynaud, G. W. Brudvig, R. H. Crabtree and O. Eisenstein, Dalton Trans., 2009, 5989–6000 RSC.
- V. R. Pattabiraman and J. W. Bode, Nature, 2011, 480, 471–479 CrossRef CAS PubMed.
- (a) J. M. Hoerter, K. M. Otte, S. H. Gellman, Q. Cui and S. S. Stahl, J. Am. Chem. Soc., 2008, 130, 647–654 CrossRef CAS PubMed; (b) N. A. Stephenson, J. Zhu, S. H. Gellman and S. S. Stahl, J. Am. Chem. Soc., 2009, 131, 10003–10008 CrossRef CAS PubMed.
- M. Zhang, S. Imm, S. Bähn, L. Neubert, H. Neumann and M. Beller, Angew. Chem., Int. Ed., 2012, 51, 3905–3909 CrossRef CAS PubMed.
- (a) C. L. Allen, B. N. Atkinson and J. M. J. Williams, Angew. Chem., Int. Ed., 2012, 51, 1383–1386 CrossRef CAS PubMed; (b) B. N. Atkinson, A. R. Chhatwal, H. V. Lomax, J. W. Walton and J. M. J. Williams, Chem. Commun., 2012, 48, 11626–11628 RSC.
- (a) M. Tamura, T. Tonomura, K. I. Shimizu and A. Satsuma, Green Chem., 2012, 14, 717–724 RSC; (b) T. B. Nguyen, J. Sorres, M. Q. Tran, L. Ermolenko and A. Al-Mourabit, Org. Lett., 2012, 14, 3202–3205 CrossRef CAS PubMed; (c) N. R. Sadu, C. M. Darapaneni and A. Subbarayappa, Org. Lett., 2013, 15, 1496–1499 CrossRef PubMed.
- R. Vanjari, B. K. Allam and K. N. Singh, RSC Adv., 2013, 3, 1691–1694 RSC.
- R. Vanjari, B. K. Allam and K. N. Singh, Tetrahedron Lett., 2013, 54, 2553–2555 CrossRef CAS PubMed.
- (a) W. Liu and J. T. Groves, Angew. Chem., Int. Ed., 2013, 52, 6024–6027 CrossRef CAS PubMed; (b) W. Liu, X. Huang, M. J. Cheng, R. J. Nielsen, W. A. Goddard III and J. T. Groves, Science, 2012, 337, 1322–1325 CrossRef CAS PubMed; (c) P. Saisaha, J. W. de Boer and W. R. Browne, Chem. Soc. Rev., 2013, 42, 2059–2074 RSC; (d) Y. Wang, H. Kobayashi, K. Yamaguchi and N. Mizuno, Chem. Commun., 2012, 48, 2642–2644 RSC.
- S. J. Smith, M. J. Riley, C. J. Noble, G. R. Hanson, R. Stranger, V. Jayaratne, G. Cavigliasso, G. Schenk and L. R. Gahan, Inorg. Chem., 2009, 48, 10036–10048 CrossRef CAS PubMed.
- L. M. Wu, H. B. Teng, X. C. Feng, X. B. Ke, Q. F. Zhu, J. T. Su, W. J. Xu and X. M. Hu, Cryst. Growth Des., 2007, 7, 1337–1342 CAS.
- J. R. Dilworth, J. Hyde, P. Lyford, P. Vella, K. Venkatasubramaman and J. A. Zubieta, Inorg. Chem., 1979, 18, 268–274 CrossRef CAS.
- H. Tsubaki, S. Tohyama, K. Koike, H. Saitoh and O. Ishitani, Dalton Trans., 2005, 385–395 CAS.
- V. P. Singh, S. Singh, D. P. Singh, P. Singh, K. Tiwari, M. Mishra and R. J. Butcher, Polyhedron, 2013, 56, 71–81 CrossRef CAS PubMed.
- A. B. P. Lever, Inorganic Electronic Spectroscopy, Elsevier, Amsterdam, 1984 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details and spectral data. CCDC 885263 and 957204. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c3ra45176c |
| This journal is © The Royal Society of Chemistry 2014 |