Emerging molecular design strategies of unsymmetrical phthalocyanines for dye-sensitized solar cell applications
Varun Kumar Singh
,
Ravi Kumar Kanaparthi
and
Lingamallu Giribabu
*
Inorganic & Physical Chemistry Division, CSIR-Indian Institute of Chemical Technology and CSIR-Network Institutes for Solar Energy (CSIR-NISE), Tarnaka, Hyderabad 500007, India. E-mail: giribabu@iict.res.in; Fax: +91-40-27160921
First published on 15th November 2013
Abstract
In recent years, dye-sensitized solar cells (DSSCs) have emerged as one of the possible solutions for the global energy crisis. Among the various components of DSSCs, the sensitizer, which harvests solar energy and injects electrons in to the semiconductor layer, plays a crucial role in achieving high efficiency and durability of the cell. To date ruthenium(II) sensitizers exhibit high efficiencies (>11.5%), but they are not so suitable for roof-top/commercial applications mainly because of their limited harvesting capability, expensive ruthenium metal and low durability. In this context, various sensitizers which include porphyrins, phthalocyanines and metal-free organic dye sensitizers have been developed and some of them were found to exhibit enhanced efficiencies compared to classical ruthenium(II) sensitizers. Man-made tetrapyrrolic systems, phthalocyanines (Pcs) have also been studied significantly because of their unique thermal and electronic properties in the red and near-IR regions. Over the years, the efficiency of Pc-based DSSC has improved to 6.1% by synthesizing various Pc derivatives and optimizing fabrication parameters. However, in the present review article, only the recent developments in Pc-based DSSCs have been documented in detail. This review also emphasizes different molecular engineering approaches that the researchers developed for achieving higher efficiency.
1. Introduction
Meeting the world's growing energy demand is one of the most significant challenges in modern human society. Today, a major share of energy produced for mankind comes from fossil fuels. While the fossil fuels are declining with a greater rate, greenhouse gases (SO2, CO2, NO2 etc.) are increasing as a result of fossil fuels and causing severe global warming. So, there is a great demand and urgency for new technologies that are economically feasible and environmental friendly. Among the many renewable energy sources, solar energy is the best for electrical power generation as it is abundant, clean, and viable. Therefore, if we could accomplish harvesting merely a tiny fraction of the solar energy that reaches the planet earth, we would solve many of our problems not only in energy, but also global, environmental and political issues.A solar cell is a device that directly converts light energy into electrical energy and it works on the photovoltaic effect. The first practical photovoltaic cell was developed in 1954 at Bell Laboratories based on elemental silicon with 6% efficiency.1 Since then, a great variety of photovoltaic devices have been developed and some of them are already proven to show good power conversion efficiency. As a whole, solar cells are broadly categorized into three generations.2–4 The first two generations are mainly based on crystalline and multi-crystalline silicon (c-Si), amorphous silicon (a-Si), Cd–Te, Ga–As and CIGS etc. The materials that are associated with the first two generations of solar cells are either hazardous or not cost effective. In order to overcome the drawbacks of first and second generation solar cells, scientists have developed excitonic solar cells, tandem solar cells etc., which are categorized as third-generation solar cells. The excitonic solar cells are broadly divided into nanocrystalline, dye-sensitized solar cells (DSSCs) and organic/polymer solar cells.
Dye-sensitized solar cells are the most promising alternative to conventional solar cells emerging dramatically in recent years. These DSSCs work under entirely different principles compared to the conventional solar cells and their mechanism is close to natural photosynthesis. Unlike the conventional solar cells (silicon p–n junction cells), in DSSCs interfacial processes play a vital role. Conventional solar cells are also known as minor carrier devices in which their efficiency is determined by the ability of photogenerated minor carriers (for example electrons in a p-type material) to escape from one side of the device before recombination with the major carriers. Thus, the minor carriers' properties such as lifetime and diffusion length are essential parameters of the device. Even though the interfaces are important in these p–n junction devices, the charge carrier processes of photogeneration, separation and recombination occur primarily in bulk material which determines the ultimate power conversion efficiency. Therefore, bulk semiconductor properties such as crystallinity and chemical purity often control the efficiency of conventional solar cells and unfortunately optimizing these properties is more expensive and arduous. On the other hand, DSSCs belong to major carrier devices in which electrons are found exclusively in one phase and holes in another phase. Charge carriers are generated at the interface between the electron-conducting and hole-conducting phases via exciton dissociation. In other words, all important charge carrier processes like photogeneration, separation and recombination occur primarily or exclusively at the interface. Henceforth, the interfaces properties are of paramount important and bulk properties are less critical. As a result, the investigated materials do not need to be highly pure which apparently reduces the cost of overall device fabrication. These differences between DSSCs and p–n junction solar cells provide an opportunity to work on various essential requirements of the DSSCs for better understanding and improving the present state of art and perhaps for these reasons it is believed that among many research areas, the study of DSSCs is a fruitful topic.
O'Regan and Gratzel were the first to report the DSSC concept in 1991 with a remarkable efficiency of 7.1% based on natural photosynthesis.5 Over the last twenty years, the DSSCs efficiency improved further and recently it has reached 12.3%.6 Among many other components of DSSC devices, the sensitizer is known to play a crucial role in achieving high efficiency and durability of the device. Extensively studied sensitizers are Ru(II) polypyridyl complexes with the general structure ML2(X)2 where L stands for 2,2-bipyridyl-4,4′-dicarboxylic acid, M is Ru, and X represents thiocyanate (–NCS). In fact, the ruthenium complex cis-RuL2(NCS)2, N3 dye has become a paradigm of heterogeneous charge-transfer sensitizers for mesoporous DSSCs.7 The high-energy conversion efficiency of this dye is generally attributed to the ultra-fast electron injection from dye to the TiO2 conduction band and a much slower reverse process. A number of modifications have been made in order to further improve the efficiency and durability of the device.8–12 Even though ruthenium(II) complexes are found to be good in terms of power conversion efficiency, they have certain drawbacks: ruthenium sensitizers are very expensive due to the rarity of the metal, several difficult synthetic protocols, lack of absorption and lower molar extinction coefficients in the red region of the absorption spectrum often limit their broad utility in the manufacturing of prototype DSSCs panels and thereby commercialization of the technology. In order to overcome these problems, a great variety of alternative sensitizers has been studied for the last two decades based on non-ruthenium metal complexes, metal-free organic sensitizers, tetrapyrrolic compounds which include porphyrins, phthalocyanines and corroles etc.13–28 The present review’s emphasis is mainly on recent developments and emerging trends of Pc-based sensitizers for DSSC applications. Although few review articles have appeared on Pcs and their application in solar cells, for last two years, several research papers have been published in the literature which shows their importance in the DSSCs research. Therefore, we reflect to document these recent emerging trends in such a way that this article enables the scientific community to understand the current state of art and how to improve the power conversion efficiency of the phthalocyanine sensitizers further.
2. Working principle
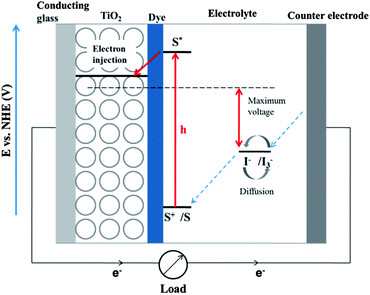
The basic working principle and essential requirements of DCCS are well documented in the literature.29–31 The DSSC is composed of a dye/sensitizer anchored to nanocrystalline mesoporous TiO2 film on conducting glass (working electrode), electrolyte, spacer in between two electrodes, platinum (Pt) sputtered conducting glass (counter electrode) and a cell sealant. Fig. 1 presents a schematic energy diagram of a DSSC and working principle. The dye photosensitizer anchored to the TiO2 absorbs incident photon flux and is excited from the ground state owing to its MLCT transition. Then the excited dye injects electrons into the conduction band of TiO2 and results in oxidation of the sensitizer. The injected electrons in the conduction band of the TiO2 film are transported through the surface or the interconnected TiO2 nanoparticles by diffusion towards the back contact (conducting FTO glass) and consequently they reach the counter electrode through external load.The oxidized photosensitizer is regenerated by accepting electrons from the redox couple (e.g. I−/I3− in acetonitrile). I− ion regenerates the dye photosensitizer to its ground state by giving one electron and is apparently itself oxidized to I3− ion. Then the oxidized I3− ion diffuses to the counter electrode (Pt-coated FTO glass plate) and is reduced back to I− ion. The DSSC performance is predominantly judged based on four energy levels: ground state (HOMO) and excited state (LUMO) of the sensitizer, Fermi level of TiO2 (located near the conduction band level) and the redox potential of the redox couple (I−/I3−) in the electrolyte solution. The photocurrent generated from the DSSC is determined by the energy difference between the HOMO and LUMO of the photosensitizer corresponding to the band gap of the semiconductor, TiO2. The smaller the HOMO–LUMO gap, the larger the photocurrent generation because of utilization of the long-wavelength region of the solar radiation. The LUMO of photosensitizer must be sufficiently negative with respect to the conduction band of TiO2 in order to inject electrons effectively. In addition, substantial electronic coupling between the LUMO and the conduction band of TiO2 is also essential for effective electron injection thus to improve the overall DSSC efficiency.
3. Symmetrical phthalocyanine sensitizers
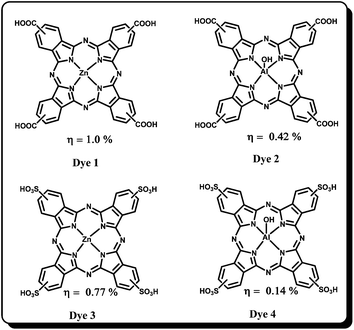
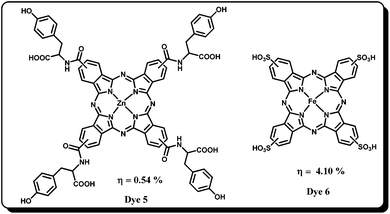
Phthalocyanines are tetrapyrrolic cyclic aromatic (18-π electron) organic molecules, demonstrated as potential candidates for several practical applications that include optoelectronic, photocatalysis, chemical sensors, photodynamic therapy and photodegradation of organic pollutants etc. owing to their unique optical and electronic properties.32–37 In general, phthalocyanines are well characterized by an intense Soret-band (∼350 nm) and Q-band (∼650–700 nm) with molar absorptivity (ε) values that exceed 105 L mol−1 cm−1, which will be interesting features for solar-cell applications. Pcs exhibit good absorption in the red and/or near-IR region of the solar spectrum and they can be tuned to be transparent over a large region of the visible spectrum, thereby enabling the possibility of using them as “photovoltaic windows”: a red/near-IR absorbing photovoltaic cell. In place of a window, one can place this photovoltaic window which allows visible light to enter a building while harvesting the red/near IR part of solar energy. Moreover, with this kind of photovoltaic window, solar heating of buildings can be reduced or avoided completely. The thermal and electronic properties of Pcs are suitable for the sensitization of wide band-gap semiconductor oxides such as TiO2, SnO2 etc. The optical, thermal and physical properties of Pcs can be tuned by introducing various substituents at peripheral and non-peripheral positions as well as by introducing various metals at its central cavity. However, the Pcs often exhibit low power conversion efficiencies due to poor solubility in common organic solvents, aggregation due to the planar Pc macrocycle and lack of electron directionality in its excited state. In 1999, for the first time, Nazeeruddin et al. reported zinc(II) (Dye 1 and 3) and aluminium(III) (Dye 2 and 4) symmetrical Pcs bearing carboxylic acid and sulfonic acid anchoring groups to facilitate proper adsorption on to the TiO2 surface (Fig. 2).38 Among these dyes the zinc(II) tetracarboxy phthalocyanine showed maximum overall power conversion efficiency of 1% and the IPCE values are between 10% and 45%.The low efficiencies of these Pcs were attributed to deactivation of the excited Pc because of their high aggregation originated from the planar configuration. Since then many efforts appeared in the literature which deal with reducing the aggregation and thereby increasing the efficiency. Aranyos et al. have reported a series of Pcs with aryl groups connected at the peripheral positions of the Pcs.39 Interestingly, even though these Pcs sensitizers do not have any anchoring group they have shown reasonable IPCE values, 4–9%.A few hyper-branched zinc(II) phthalocyanines have also been synthesized and nearly 67% IPCE was observed, but their overall power conversion efficiencies are still restricted to 1%.40 The main reason for the low performance of these Pc-based DSSC was found to be due to aggregation which promotes non-radiative deactivation of the dye excited states in aggregates. Later He et al.41 reported a zinc(II) phthalocyanine bearing tyrosine substituents (Dye 5) in order to increase solubility and to reduce aggregation at the semiconductor surface (Fig. 3). However, this dye showed merely 0.54% efficiency with an IPCE of 24%, attributed to the fast recombination of injected electrons and insufficient steric hindrance of the tyrosine moiety. In another study, myristic acid was employed as a co-adsorbent of aluminium(III) tetraphenoxy phthalocyanine hydroxide for the sensitization of nanocrystalline TiO2.42,43 It was found that the co-adsorbent showed a dramatic decrease in the aggregation of Pc on the nanocrystalline surface and as a result the test cell efficiency was improved. Balaraju et al.44 have used iron tetrasulfonic acid phthalocyanine (Dye 6) sensitizer and the corresponding device has shown an overall conversion efficiency of 4.1% by employing a PEDOT:PSS-coated FTO counter electrode instead of the usual Pt counter electrode and dilute HNO3 treatment of TiO2. The increase in power conversion efficiency of the DSSC based on nitric acid treatment for the photo-electrode is mainly attributed to the increase in photocurrent. A comparative photovoltaic investigation of DSSCs using HCl-treated TiO2 photo-electrode indicates that the HNO3-treated photo-electrode retards back electron transfer at the interface with electrolyte and increases the amount of dye. In a recent work, Jin and co-workers have reported zinc(II) phthalocyanine with eight octacarboxylic acid anchoring sites. Although the aggregation is reduced, the DSSC device was not found be efficient.45
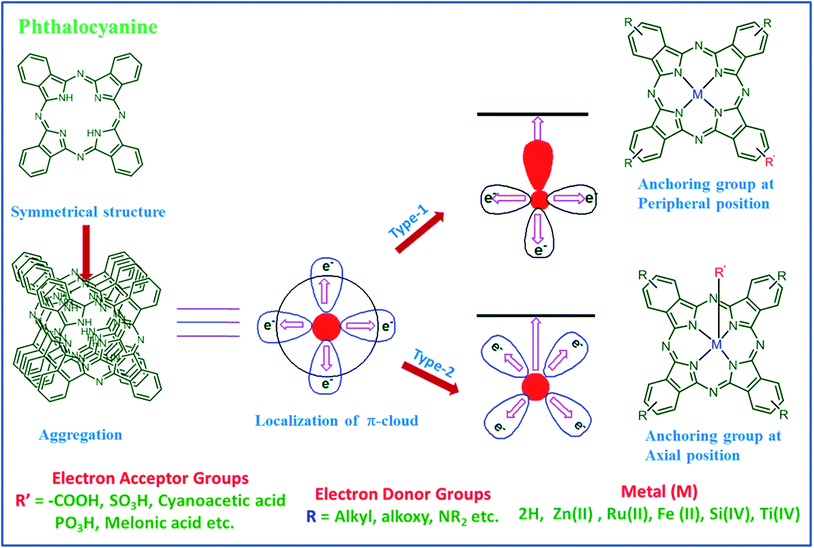
In order to further improve the efficiency one has to minimize the aggregation of a Pc macrocycle through molecular engineering. There are two methods to minimize the aggregation of phthalocyanine macrocycles. One is the substitution of anchoring or aromatic groups at axial position(s) of the resident metal ion of the phthalocyanine. A second method is introduction of bulky substituents as well as anchoring groups at peripheral positions i.e., ‘push–pull’ concept (Fig. 4).
4. Axially substituted phthalocyanines
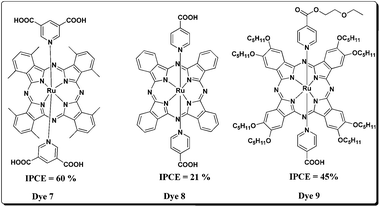
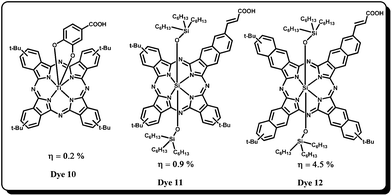
Nazeeruddin and co-workers tried to overcome the aggregation problem by synthesizing axially substituted Pcs and reported ruthenium(II) phthalocyanine sensitizer, Dye 746 bearing bis(3,4-dicarboxy)pyridine as anchoring groups (Fig. 5). The sensitizer 7 had shown a promising 60% IPCE in the near IR region, however its overall efficiency was not impressive due to desorption of sensitizer molecules on the TiO2 surface because of weak the coordinating ability of the Ru(II) metal with the pyridine ligand. Yanagisawa et al. have compared the sensitization properties of an axially substituted anchoring phthalocyanine with that of peripherally substituted phthalocyanines under identical test cell conditions.47 The axial substituted sensitizer, Dye 8 has shown better efficiency (0.61%) than the peripherally substituted counterpart sensitizer Dye 9 (0.58%) and the decreased efficiency was attributed to reduced stability of adsorbed Pcs on the TiO2 surface. As the axially substituted Pcs are showing somewhat better efficiencies (owing to their reduced aggregation and near-IR region absorption) Durrant, Morandiera et al. have studied interfacial electron transfer dynamics. They demonstrated that ultra-fast electron transfer rates are not required for efficient electron injection yield. Afterward Morandeira et al. studied the effect of co-adsorbents on the device performance of axially substituted ruthenium(II) phthalocyanies in order to understand the DSSC device physical aspects. The effect of lithium cation (Li+) and co-adsorbent chenodeoxycholic acid (CHENO) was studied systematically.48 From the previous studies it is known that the addition of Li+ is expected to lower the conduction band edge of TiO2 and decrease the photovoltage as well. However, an optimum concentration of Li+ increases the electron injection yield and photocurrent of the DSSC device. Interestingly, it was observed that Ru-phthalocyanine sensitizers showed a remarkable decrease in the aggregation compared to symmetrical phthalocyanines sensitizers reported till that time. On the other hand, the co-adsorbent CHENO has also shown a significant effect on the device performance. The CHENO hinders the formation of aggregates and thereby enhances the electron injection yield and ultimately increases the photocurrent response. Moreover, it is also found that recombination (dye cations with the electrons in TiO2) becomes slower and regeneration of the dye cations by the redox electrolyte is faster which apparently enhanced the photocurrent. The reduced recombination can also due to increased hole–electron (phthalocyanine ring–TiO2 surface) distance because of the unique geometry of CHENO which helps it to stand straight on the TiO2 surface thereby slowing down the dye cation–electron recombination.Torres and co-workers have utilized the axial position of titanium(IV) phthalocyanines, (Dye 10) for DSSC applications (Fig. 6).49 Unlike Gratzel and co-workers, they have connected anchoring carboxy catechol at the axial position. By using covalent bonds, desorption from the surface of nanocrystalline TiO2 can be minimized. The solution absorption spectrum of Dye 10 exhibits an intense Q-band (λmax = 702 nm, ε = 135![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 dm3 mol−1 cm−1) and the dye is strongly adsorbed on to the TiO2 surface. However, its photovoltaic properties (IPCE = 19% and η = 0.2%) are not impressive due to poor electron injection from the LUMO of the phthalocyanine to the TiO2 conduction band. Axially substituted Pcs such as Si(IV) phthalocyanines have also been reported and tested for their applicability in DSSCs. In this context, it is worthwhile to discuss a recent paper by Sellinger and Gratzel in which Dye 11 and Dye 12 have been shown to be very good candidates for DSSCs.50 The silicon naphthalo–phthalocyanine hybrid sensitizer, Dye 12 showed excellent light harvesting efficiency (IPCE = 80%) and high photocurrent density of 19 mA cm−2. Bulky tert-butyl substituents were added to the core periphery in order to avoid aggregation and trihexylsiloxy groups were introduced as coordinating ligands to the silicon center. The sensitizer Dye 12 has shown a Jsc of 19 mA cm−2, open circuit voltage, Voc = 0.46 V, fill factor (FF) = 0.51 and overall efficiency of 4.5% under standard irradiation conditions. But, the sensitizer Dye 11 has shown a lower efficiency of 0.9% which was attributed to insufficient driving forces for electron injection into the conduction band of TiO2 and regeneration of the oxidized dye by the redox electrolyte. Although there are a few other Si(IV) phthalocyanines in the literature, their photovoltaic behaviour is not efficient.
000 dm3 mol−1 cm−1) and the dye is strongly adsorbed on to the TiO2 surface. However, its photovoltaic properties (IPCE = 19% and η = 0.2%) are not impressive due to poor electron injection from the LUMO of the phthalocyanine to the TiO2 conduction band. Axially substituted Pcs such as Si(IV) phthalocyanines have also been reported and tested for their applicability in DSSCs. In this context, it is worthwhile to discuss a recent paper by Sellinger and Gratzel in which Dye 11 and Dye 12 have been shown to be very good candidates for DSSCs.50 The silicon naphthalo–phthalocyanine hybrid sensitizer, Dye 12 showed excellent light harvesting efficiency (IPCE = 80%) and high photocurrent density of 19 mA cm−2. Bulky tert-butyl substituents were added to the core periphery in order to avoid aggregation and trihexylsiloxy groups were introduced as coordinating ligands to the silicon center. The sensitizer Dye 12 has shown a Jsc of 19 mA cm−2, open circuit voltage, Voc = 0.46 V, fill factor (FF) = 0.51 and overall efficiency of 4.5% under standard irradiation conditions. But, the sensitizer Dye 11 has shown a lower efficiency of 0.9% which was attributed to insufficient driving forces for electron injection into the conduction band of TiO2 and regeneration of the oxidized dye by the redox electrolyte. Although there are a few other Si(IV) phthalocyanines in the literature, their photovoltaic behaviour is not efficient.
5. Unsymmetrical phthalocyanine sensitizers for DSSCs
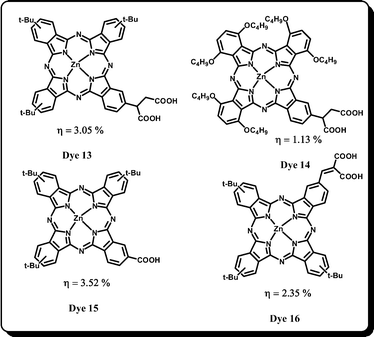
Based on many reports, symmetrical Pcs have indeed been shown to be potential sensitizers for DSSC applications. Although their synthesis and purification are not a matter of concern, the majority of them are inefficient in achieving high power conversion efficiencies mainly due to poor solubility in common organic solvents, aggregation and lack of directionality in the excited state. Hence, it was realized that in order to improve efficiency, one has to modify the molecular structure of the existing symmetrical Pcs. By placing appropriate substituents on the Pcs, one can tune desired properties such as solubility and aggregation. Moreover, it is indeed possible to break the symmetry of Pcs by placing two different functional groups on the molecule. This can be achieved by mixed condensation of different substituted phthalonitriles and desired the Pc can be synthesized in such a way that it should have an acid substituent at one of the isoindoline units for better binding with the TiO2 surface. For their potential applications as photosensitizers, the other three isoindoline units can be functionalized with different substituents to make them more soluble, facilitate ease of purification and hinder aggregation which has an undesirable effect in its DSSC application. Then the obtained precursors are reacted together and the resulting products can be carefully purified by column chromatography.In 2004, Yanagisawa and co-workers reported two unsymmetrical phthalocyanines with different metal ions at the phthalocyanine's central cavity.51 The zinc-Pc-based sensitizer showed an overall conversion efficiency of 0.03% whereas Ru(II) phthalocyanine sensitizer showed 0.4% efficiency. The low efficiency of these phthalocyanines was attributed to the rupture in the conjugation between the macrocycle and the anchoring group which resulted in poor electron injection from the excited state of the phthalocyanine to the TiO2 conduction band. Later, in order to further improve the efficiency of DSSC devices, unsymmetrical phthalocyanines have been designed and developed based on the “push–pull” molecular architecture. The first breakthrough appeared in 2007 by Giribabu and co-workers in which they reported a very interesting unsymmetrical phthalocyanine sensitizer (Dye 13) which gave the highest overall conversion efficiency of 3.05% using the iodide–triiodide electrolyte (Fig. 7).52–54 The Dye 13 sensitizer was designed in such a way that it has three bulky tert-butyl groups to increase solubility and to decrease aggregation. The tert-butyl group also acts as an electron-releasing (push) group and the succinic acid group serves as an electron-withdrawing group (pull) to anchor with the TiO2 surface. The Dye 13 sensitizer showed an excellent IPCE of 75% and its high efficiency was attributed to the excited state directionality thereby providing efficient electron injection. By using a durable redox electrolyte (high boiling point solvents such as γ-butyrolactone) the Dye 13 sensitizer showed ∼2% conversion efficiency and the device was found to be stable for at least 1000 hours.54 The Dye 13 sensitizer gives an IPCE of 43% and overall conversion efficiency of 0.87% by using solid-state organic hole transporter 2,2′,7,7′-tetrakis(N,N-di-p-methoxyphenylamine)-9,9′-spirobifluorene (spiro-MeOTAD) instead of liquid electrolyte. Subsequently, Giribabu and co-workers53 have designed and synthesized a stronger “push–pull” derivative Dye 14 and its photovoltaic performance was compared with Dye 13. The new sensitizer Dye 14 possesses six donor butoxy groups at the α-position due to which the absorption spectrum shifted by ∼10 nm compared to Dye 13. But, unfortunately, Dye 14 sensitizer gives only 1.13% overall efficiency and 25% IPCE. The prime reason for the low efficiency was poor electron injection quantum yield which also resulted in the decreased Jsc value.
In another study by Torres and co-workers, Dye 13 was redesigned having only one carboxylic acid group instead of two acid anchoring groups. The DSSC device fabricated using Dye 15 sensitizer showed improved overall efficiency of 3.5% under standard irradiation conditions and the IPCE value at the Q-band absorption maxima was found to be 80%.55 The reason for enhanced efficiency of Dye 15 is that the anchoring group directly attached to the phthalocyanine macrocycle, which did not break the aromaticity as was the case in Dye 13. Later, Giribabu and co-workers reported Dye 1656 sensitizer by redesigning the parent sensitizer Dye 13 based on the “push–pull” architecture and extended the π-conjugation concept. Malonic acid is the anchoring group in Dye 16. Although, the sensitizer Dye 16 exhibited better light harvesting ability (broader absorption) compared to the parent sensitizer, the overall conversion efficiency (2.35%) was not improved as expected; under similar test cell conditions Dye 13 has shown a conversion efficiency of 2.80%. The reason is that the IPCE of Dye 16 was only 47%, whereas Dye 13 has shown 71%.
Despite the fact that several researchers are working on the development of phthalocyanine-based sensitizers, the efficiency has reached little higher than 3% under standard conditions. These sensitizers are highly attractive candidates because of their high absorption coefficients but still their efficiencies are not enough for commercialization. Therefore, a great deal of research still needs to be done for understanding the device physics and performance of the unsymmetrical sensitizers.
6. Molecular design optimization: study based on unsymmetrical tert-butyl phthalocyanines
Although in many studies co-adsorbents have been employed as aggregation controllers in DSSC devices none of them have proven helpful in improving the 3% efficiency. So, in later studies of electron injection, recombination lifetime of sensitizers has become a major concern in achieving high efficiency. In this context, a few studies by Torres and coworkers57 on unsymmetrical Pcs sensitizers for DSSC applications are noteworthy to mention at this point of discussion. For example, they have designed and synthesized unsymmetrical zinc-phthalocyanines based on the “push–pull” molecular arrangement in which bulky tert-butyl donor groups and carboxylic acid anchoring group are linked to the core phthalocyanine ring through different spacers. The DSSC devices fabricated with these “push–pull” phthalocyanines reveal that the nature of the spacer unit and distance between the phthalocyanine core and the anchoring group play a significant role in determining the efficiency of the device. It was found that Dye 15 (without a spacer unit) has the highest overall power conversion efficiency (η = 3.52%) compared to Dye 17 (η = 0.4%) possessing a flexible and unconjugated pentoxy group spacer (Fig. 8). Dye 20 possessing a phenoxy spacer has also shown merely 0.67% conversion efficiency. Based on the results, it was proposed that the loss of directionality in the excited state led to poor electron coupling of the donor with titanium 3d-orbitals and thus shows remarkably low efficiencies. Further it was found that due to the flexible and non-directional nature of the oxygen linker (pentoxy and phenoxy), there is a possibility that the sensitizer might not be able to stand straight on the TiO2 surface. However, on other hand Dye 18 and Dye 19 have shown comparable efficiencies with Dye 15. Even though Dye 18 shows faster recombination dynamics (compared to the other sensitizers), it showed better efficiency (η = 2.2%) because of its high electron injection yield owing to improved directionality in the excited state.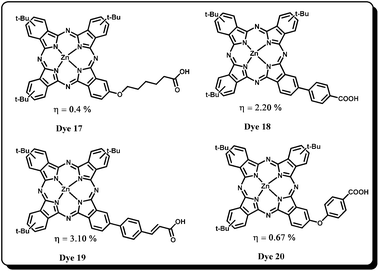 | ||
| Fig. 8 Molecular structures of unsymmetrical tert-butyl phthalocyanines with different anchoring groups. | ||
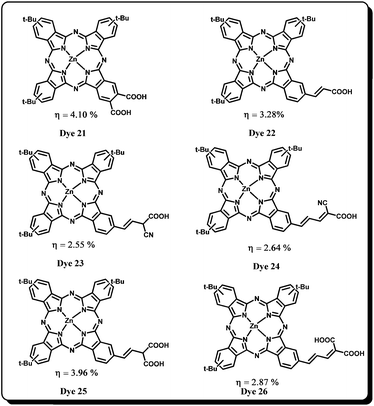
In another study, they demonstrated the effect of the anchoring group on the stability of DSSC and the device performance.58,59 Pc Dye 21 was designed to possess two carboxylic acid anchoring groups in order to improve directionality in the excited state and its efficiency in the presence of volatile and non-volatile electrolytes was compared with Dye 15 (Fig. 9). Dye 21 has shown improved conversion efficiencies of η = 3.33% and η = 4.10% in the presence of volatile and non-volatile electrolytes, respectively. Broad absorption and enhanced photocurrent response of Dye 21 are the governing factors for its higher efficiency. This major breakthrough a couple of years after the Dye 15 report had further sparked many researchers to design and synthesize Dye 22, Dye 23, Dye 24, Dye 25 and Dye 26 sensitizers (by modifying anchoring groups) in order to explore the factors governing injection efficiency and the device performance has been compared with the parent Dye 15. When a double bond was incorporated between the donor macrocycle and the anchoring carboxylic acid (Dye 22), a small decrease in IPCE was observed and the overall conversion efficiency dropped down to η = 3.28%. Then in Dye 23 and Dye 24, the cyanoacrylic acid group was used as the anchoring group. These sensitizers showed inferior device performance compared to the parent dye. In Dye 23, a lower Jsc of 5.88 mA cm−2, Voc of 587 mV and η = 2.55% was observed. The extended conjugation between phthalocyanine and cyanoacrylic acid (Dye 24) was also not helpful in achieving better efficiency (Jsc = 6.80 mA cm−2, Voc = 576 mV and η = 2.64%). However, improved photovoltaic behaviour was observed in case of Dye 25 sensitizers in which cyanoacrylic acid anchoring group was replaced with a malonic acid anchoring group. Enhanced photocurrent response (Jsc of 9.15 mA cm−2) and a slight decrease in the photovoltage were observed. The decrease in photovoltage response was attributed to low electron lifetime and an increase in the number of carboxylic groups. The DSSC device has shown overall conversion efficiency of 3.96% which is higher than the Dye 15 sensitizer. However, when the conjugation was extended between the malonic acid anchoring group and the macrocycle in Dye 26, a sudden breakdown in the Jsc = 6.86 mA cm−2, Voc = 584 mV and thus overall conversion efficiency 2.87% was observed. It was concluded that the sensitizers bearing malonic acid anchoring group could possibly be the choice of anchoring group for future designs. The efficiencies exhibited by all the sensitizers revealed the significant involvement of high order directionality in the excited state of the dye which is a prerequisite for good electronic coupling between the excited state of the dye and the titanium 3d-orbitals.
 | ||
| Fig. 9 Molecular structures of unsymmetrical tert-butyl phthalocyanines with different anchoring groups. | ||
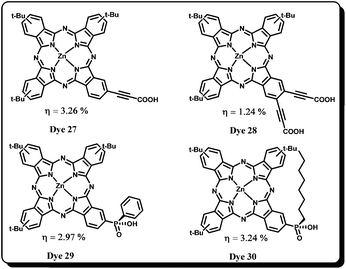
Nazeeruddin, Torres et al. examined the effect of an ethynyl spacer between the carboxylic acid anchoring group and the phthalocyanine. It is expected that due to rigid planar spacer, the phthalocyanine core will be perpendicular and closer to the TiO2 surface which enhances the electronic coupling between the dye and Ti-3d orbital. In order to understand the ethynyl effect, Dye 27 and Dye 28 have been designed and synthesized (Fig. 10).60 While Dye 27 gives a 3.26% overall conversion efficiency (IPCE = 68%), Dye 28 exhibits only ∼1% efficiency (IPCE = 22%). The higher efficiency with one carboxyethynyl spacer was attributed to the longer electron lifetime and higher short circuit current density. However, the drop in the efficiency on introduction of an additional carboxyethynyl spacer was due to the reduced Jsc owing to low driving force for charge injection from excited dye into the TiO2 conduction band.
Gratzel and co-workers61 have synthesized two new phthalocyanine sensitizers Dye 29 and Dye 30 by employing a phosphinic acid anchoring group and their photovoltaic properties were compared with Dye 15. It was observed that Dye 29 has shown a lower IPCE of 36%, attributed to the poor adsorption of Dye 29 on mesoporous semiconductor film. It was found that the phosphinic acid anchoring groups enhance long term photostability of the dye compared to the carboxylic acid anchoring group. However, their overall efficiencies were found to be slightly lower because of the decrease in the dye adsorption on the semiconductor surface. However, the sensitizers, Dye 29 and Dye 30 have shown superior conversion efficiency to Dye 15 in the presence of co-adsorbent, CDCA.
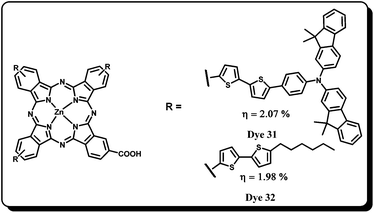
The phthalocyanines have high absorption coefficients in the near-IR (NIR) region. However, they lack absorption in the 400–600 nm region. So, by synthesizing panchromatic (absorption in the UV-Vis region) Pc sensitizers one can indeed improve their efficiencies even further.62 For this reason, Torres and Nazeeruddin have developed conjugated panchromatic sensitizers by covalently attaching an organic chromophore at three positions which has absorption in the complimentary region with the Pc core and the carboxylic acid group used for anchoring on to the TiO2 nanoparticles.63 In Dye 31, the peripheral positions are substituted with triarylamine-terminated bisthiophene units and in Dye 32, hexylbisthiophene units are attached (Fig. 11). Unfortunately, both the sensitizers have shown only 40% IPCE and ∼3% overall conversion efficiency by using a co-adsorbent. The reduced efficiencies of these dyes have been assigned to the aggregation and poor electron injection efficiencies.
7. Sterically crowded unsymmetrical phthalocyanine sensitizers
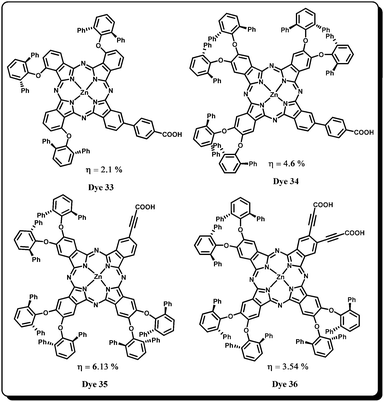
The peripheral substitution of phthalocyanines is one of the promising ways to reduce planarity and thus aggregation and it is possible to manipulate the physicochemical properties as well. Several researchers have reported phthalocyanines with long alkyl chains or bulky substituents to suppress the intermolecular interaction and thereby aggregation.64–66 The most viable way of suppressing aggregation is to have bulky substituents at adjacent positions like β,β′-positions of the phthalocyanine periphery. In fact a similar strategy has also been used before in designing various phthalocyanine sensitizers. For example, the good photovoltaic behaviour of Dye 13 and Dye 15 was due to a significant decrease in aggregation and enhanced directionality in the excited state owing to the presence of bulky tert-butyl groups at the periphery of the phthalocyanine. It was found that these dyes have performed even better in the presence of co-adsorbents like CDCA which demonstrates that there is adequate room for reducing the aggregation tendency of these molecules. For the first time Imahori and coworkers reported a substituted phthalocyanine (β,β′-position) synthesized for DSSCs applications.63 They synthesized free base and zinc phthalocyanines with 4-tert-butylphenyl substituents covalently attached at six β,β′-positions of the phthalocyanines and benzoic acid anchoring groups at the other two β,β′-positions. But the zinc phthalocyanine sensitized cell displayed poor power conversion efficiency of 0.57% (Jsc = 1.47 mA cm−2; Voc = 0.54 V; FF = 0.71) and 4.9% IPCE. The free base counterpart of the same substituted phthalocyanine sensitizer has shown even lower power conversion efficiency. Moreover, it was observed that the these sensitizer did not show any change in device performance even in the presence of the CDCA co-adsorbent which indeed proved the ability of such a molecular design (bulky alkyl substitutions at β,β′-positions) to reduce aggregation. Poor photovoltaic performance of these sensitizers was attributed to the small driving force for electron injection from the LUMO of free base/zinc phthalocyanines to TiO2 and lack of proper electronic coupling between the dye excited state and the TiO2 conduction band.Later Mori et al. designed a promising “push–pull” phthalocyanine sensitizer demonstrating the importance of three dimensional (3D) enlargement of the phthalocyanine molecular structure to suppress the aggregation completely. Three new sensitizers Dye 18, Dye 33 and Dye 34 were synthesized and their aggregation reducing order is Dye 34 > Dye 33 > Dye 18 (Fig. 12).67 The Dye 34 sensitizer showed a maximum IPCE of 78%, high Jsc of 10.4 mA cm−2 and record overall conversion efficiency of 4.6%. Completely suppressed aggregation, improved electron injection yield and increased directional electron-transfer behaviour were found to be the primary reasons for the record efficiency of Dye 34. In an another study, Bisquert et al.68 have studied the electron transfer dynamics based on two sterically hindered phthalocyanines (Zn(II) and free base) and compared their results with the benchmark N719 dye. These phthalocyanines possess electron-donating tert-octylphenoxy groups and the electron-withdrawing acid anhydride group served as an anchoring group. Both the sensitizers have shown lower efficiencies when compared to N719 dye. However, relatively zinc phthalocyanine gave slightly lower efficiency than that of free base phthalocyanine, attributed to the higher number of protons in free base phthalocyanine interacting with the semiconductor surface which lowers the conduction band edge. It was concluded that increasing the injection yield and lifting the energy levels of the phthalocyanine could help in improving the DSSC efficiency. The photovoltaic data of all phthalocyanines are summarized in Table 1.
| Sensitizer | IPCEb (%) | Jscb (mA cm−2) | Vocb (V) | FFb | ηb (%) |
|---|---|---|---|---|---|
| a Redox electrolyte is I−/I3− in volatile organic solvent.b IPCE: Incident photon to current conversion efficiency; Jsc: current density; Voc: open circuit voltage; FF : Fill factor; η : Efficiency.c IPCE not reported.d Photovoltaic data not reported.e Counter electrode is PEDOT:PSS, otherwise Pt counter electrode. | |||||
| Dye 1 | 43 | 5.40 | 0.416 | — | 1.00 |
| Dye 2 | 13 | 1.10 | 0.466 | — | 0.42 |
| Dye 3 | 30 | 0.48 | 0.453 | — | 0.77 |
| Dye 4 | 10 | 0.60 | 0.382 | — | 0.14 |
| Dye 5 | 24 | 2.25 | 0.360 | 0.670 | 0.54 |
| Dye 6 | —c | 6.94 | 0.940 | 0.630 | 4.10e |
| Dye 7 | 60 | —d | —d | —d | —d |
| Dye 8 | 21 | 3.15 | 0.360 | 0.540 | 0.61 |
| Dye 9 | 45 | 2.61 | 0.340 | 0.650 | 0.58 |
| Dye 10 | —c | — | — | — | 0.20 |
| Dye 11 | 25 | 6.56 | 0.360 | 0.38 | 0.90 |
| Dye 12 | 80 | 19 | 0.460 | 0.51 | 4.50 |
| Dye 13 | 75 | 6.5 | 0.635 | 0.743 | 3.05 |
| Dye 14 | 25 | 2.81 | 0.525 | 0.764 | 1.13 |
| Dye15 | 80 | 7.60 | 0.617 | 0.75 | 3.52 |
| Dye 16 | 47 | 5.63 | 0.557 | 0.75 | 2.35 |
| Dye 17 | 9 | 0.90 | 0.55 | 0.72 | 0.40 |
| Dye 18 | 56 | 4.80 | 0.61 | 0.74 | 2.20 |
| Dye 19 | 65 | 6.80 | 0.613 | 0.74 | 3.10 |
| Dye 20 | 16 | 1.44 | 0.611 | 0.75 | 0.67 |
| Dye 21 | 65 | 9.37 | 0.605 | 0.725 | 4.10 |
| Dye 22 | 75 | 7.37 | 0.609 | 0.74 | 3.28 |
| Dye 23 | 50 | 5.88 | 0.587 | 0.75 | 2.55 |
| Dye 24 | 55 | 6.80 | 0.576 | 0.69 | 2.64 |
| Dye 25 | 67 | 9.15 | 0.600 | 0.72 | 3.96 |
| Dye 26 | 40 | 6.86 | 0.584 | 0.72 | 2.87 |
| Dye 27 | 70 | 7.01 | 0.610 | 0.73 | 3.26 |
| Dye 28 | 21 | 3.70 | 0.520 | 0.73 | 1.40 |
| Dye 29 | 36 | 7.07 | 0.563 | 0.75 | 2.97 |
| Dye 30 | 85 | 7.67 | 0.559 | 0.76 | 3.24 |
| Dye 31 | 40 | 5.25 | 0.541 | 0.73 | 2.07 |
| Dye 32 | 40 | 4.96 | 0.543 | 0.74 | 1.98 |
| Dye 33 | 50 | 4.8 | 0.58 | 0.77 | 2.10 |
| Dye 34 | 86 | 10.4 | 0.63 | 0.70 | 4.60 |
| Dye 35 | 85 | 1.31 | 0.585 | 0.76 | 6.13 |
| Dye 36 | 50 | 8.77 | 0.584 | 0.71 | 3.63 |
| Dye 37 | 65 | 12.8 | 0.61 | 0.68 | 5.30 |
| Dye 38 | 82 | 13.7 | 0.613 | 0.70 | 5.90 |
| Dye 39 | —c | 3.26 | 0.604 | 0.67 | 1.07 |
| Dye 40 | —c | 2.33 | 0.504 | 0.75 | 0.89 |
In another study by following a similar structural design to Dye 34, Ragoussi and co-workers60 replaced the benzoic acid anchoring group with a carboxyethynyl anchoring group and reported Dye 35 and Dye 36. The two dyes differed only in the number of carboxyethynyl groups. Dye 35 with one ethynyl spacer group has shown η = 6.1% conversion efficiency, the highest efficiency reported so far using phthalocyanines and the IPCE also reached more than 85% at 700 nm. On the other hand, Dye 36 has shown η = 3.54% and 55% IPCE. The high Voc of Dye 35 was attributed to the enhanced electron lifetime and decreased aggregation. On the other hand, the decreased efficiency of Dye 36 (compared to Dye 35) was due to its low injection efficiency, evident from the LUMO level (calculated using electrochemical techniques) of the Dye 36. These results suggests that the ethynyl group is the most optimal bridge between the Pc-core and the carboxylic anchoring group which provides good electronic coupling between the Pc and TiO2. However, the presence of two ethynyl spacer anchoring groups was found to decrease the efficiency mainly due to the decreased driving force for electron injection from the excited dye into the TiO2 conduction band.
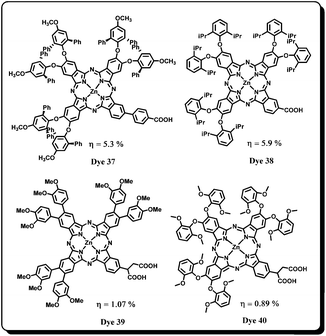
Mori, Kimura and co-workers have further explored the molecular design strategy based on 3D-enlargement by substituting methoxy groups at the peripheral positions and reported Dye 3769 and Dye 38 (Fig. 13).70 Dye 37 has better solubility and electron donation ability to the phthalocyanine core. The sensitizer Dye 37 differed only by a methoxy group on the peripheral diphenylphenol moiety compared to Dye 34. While the aromatic substituent in these two dyes were kept constant to optimize the electronic linker structure, in Dye 38 a different adsorption site (compared to Dye 37) had been introduced with a vision to improve the electronic coupling with TiO2. The sensitizer Dye 37 has shown high photocurrent response, IPCE of 72% and 5.3% conversion efficiency. It was found that the better electron donating ability of methoxy groups in 2,6-diphenyl-4-methoxyphenoxy of Dye 37 helped to increase injection yield and thus enhanced overall conversion efficiency. The structural optimization was carried out by replacing the bulky 2,6-diphenyl-4-methoxyphenoxy groups with 2,6-diisopropylphenoxy groups in Dye 38 to facilitate close packing of dye molecules on the TiO2 surface which is reflected in its photovoltaic properties. The Dye 38 showed an improved power conversion efficiency of 5.9% (Jsc = 13.7 mA cm−2, Voc = 0.613 V, FF = 0.70) when compared to Dye 37. Based on charge separation and injection efficiency, it was suggested a direct link from the carboxylic acid group to the phthalocyanine core was the appropriate choice in designing new sensitizers. Molecular modeling studies confirmed that the undesirable orientation of Dye 37 on TiO2 was the main reason for low adsorption density and a decreased efficiency. This study further revealed that the speculation “greater the steric hindrance, greater will be the efficiency” (keeping the anchoring group same) may not always be correct. The efficiencies greatly depend on the adsorption density of dye on the TiO2 surface as well.
Recently, Giribabu, Filippo, Nazeeruddin and co-workers have reported new sterically hindered phthalocyanines based on methoxy substituents having different patterns.71 The sensitizer Dye 39 possess 3,4-dimethoxyphenyl substituents at the β-positions while Dye 40 has 2,6-dimethoxyphenoxy substituents as electron donors. However, with Dye 39 only 1.07% power conversion efficiency has been achieved due to lack of directionality in the excited state. Unfortunately, the sensitizer Dye 40 which was supposed to show better efficiency than that of Dye 39 because of decreased aggregation due to comparatively hindered structure had indeed shown even lower efficiency (0.89%). On the basis of theoretical calculations, it was stated that the charge delocalization of the LUMO extends to only one of the carboxylic anchoring groups, which may decrease charge regeneration. Very recently, Giribabu and co-workers have designed an unsymmetrical zinc(II) phthalocyanine with tert-butylthiophenyl groups at the non-peripheral positions.72 Due to substitution at the non-peripheral positions the phthalocyanine has strongly red shifted absorption centered at 750 nm; the absorption in the near-infrared region can help to harvest a greater number of photons and thereby increase the cell efficiency. However, it was observed that this sensitizer could harvest a very limited amount of solar energy and the cell showed 0.40% efficiency.
8. Co-sensitization effects in unsymmetrical phthalocyanine based DSSCs
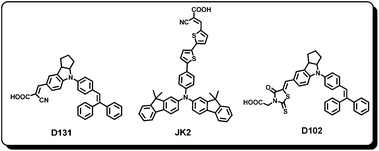
Over the years, photovoltaic performance of phthalocyanines has improved quite significantly and in fact they have been shown to be one of the alternative sensitizers for commercializing DSSC technology. This came true only after finding proper solutions for reducing aggregation and enhancing the directionality in the dye excited state. However, there has been limited work on the improvement of phthalocyanine absorption in the 400–550 nm region, where the molar absorption coefficient of the phthalocyanine is minimal. Co-sensitization is a convincing way to enhance the device performance through a combination of two or more dyes with complimentary absorptions characteristics on the semiconductor films. For this purpose, the dyes have to be chosen in such a way that they should broaden the absorption, which essentially enhances the harvesting ability thus charge transfer efficiency and to reduce the back transfer of electrons from the interconnected semiconductor oxide nanoparticle material to the sensitizing dye. The co-sensitization strategy has been reported before employing ruthenium complex,73,74 metal-free organic dyes75,76 and porphyrin systems77,78 to show enhanced photovoltaic performance compared to their single dye systems.The first phthalocyanine–organic dye co-sensitization system for dye solar cell application was reported in 2007 by Torres and co-workers.55 They demonstrated the enhanced photovoltaic response of Dye 15 co-sensitized with a reported organic dye JK279,80 and observed enhanced device performance, Jsc = 16.20 mA cm−2, Voc = 0.66 V, FF = 0.72 and η = 7.74% compared to the individual phthalocyanine dye (3.5%) and JK2 (7.08%) (Fig. 14). The photoresponse of the cocktail system has reached up to 700 nm with incident photon to current conversion efficiency of 72% at 690 nm. The increased performance of the cocktail sensitizer was related to the high molar extinction coefficient of the dyes which would provide sufficient space on the semiconductor surface to allow absorption of another dye having complimentary absorption.
In 2012, Mori and co-workers69 co-sensitized Dye 37 with two organic dyes, D10281,82 and D13183 resulting in an enhanced photovoltaic performance of the cocktail sensitizer. The DSSC device fabricated from cocktail sensitizer Dye 37/D102 exhibited 17% enhancement in the Jsc value while Dye 37/D131 cocktail sensitizer showed 28% enhancement in the Jsc compared to Dye 37 DSSC devices. An impressive IPCE of 80% even after co-sensitization showed the capability of good electron injection of the cocktail sensitizers. There was no decrease noticed in the IPCE value in the near-IR region which also points towards zero interaction between the two dyes. The Jsc of 15.3 mA cm−2, Voc of 0.63 V, FF of 0.64 corresponding to an overall efficiency of 6.2% was achieved with the Dye 37/D131 system while the Dye 37/D102 cocktail system has shown Jsc of 13.9 mA cm−2, Voc of 0.625 V, FF of 0.64 giving conversion efficiency η = 5.6%. The authors proposed that the bulky substituents not only reduced the aggregation but were also very effective in inhibiting the interaction between the two complimentary dyes because of an increase in the distance between their molecular frameworks.
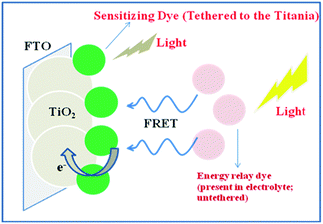
9. Energy relay dyes: a strategy to increase the light harvesting ability of phthalocyanine dyes
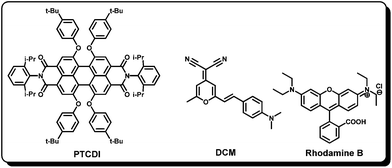
To have an efficient cocktail sensitizer based on the co-sensitization phenomena, it is necessary that the constituent dyes should adsorb strongly on the TiO2 surface, transfer charge efficiently into the TiO2, and be effective at regeneration84 and charge injection.85–88 However, so far there have been limited dyes which exhibit all these characteristics. Moreover, the co-sensitization methodology has a constraint regarding the number of anchoring sites on the TiO2 surface that are available for the dye attachment. In this context, recently, long range energy transfer has been employed to increase the light harvesting ability in DSSCs (Fig. 15).89–91Recently, Cid et al. have reported an energy relay dye using a highly fluorescent chromophore, PTCDI, dissolved in electrolyte and tested using Dye 15 (Fig. 16).55 A 28% increase in the photocurrent response was observed due to the increase in external quantum efficiency from the 400–600 nm region giving a total 26% increase in the power conversion efficiency. However, no change in open circuit potential and fill factor of the relay device was found. The device efficiency was found to be 2.55% and 3.21% in the absence and presence of PTCDI, respectively.92 In another work by Hardin and others,93 the energy relay dye 4-(dicyanomethylene)-2-methyl-6-(4-dimethylaminostyryl)-4H-pyran (DCM) was used with Dye 15 and the overall power conversion efficiency increased from 3.5% to 4.5%. In this study extremely high average excitation transfer efficiency (>96%) with transparent TiO2 films was demonstrated. However, the external quantum efficiency of the relay dye was limited to 40% in the optimized device due to low absorption of the relay dye. They have further showed that the energy relay dyes should have a high molar absorption coefficient and very good solubility in the electrolyte to achieve an optimum DSSC architecture.
In a demonstration of the enhancement in the photovoltaic performance of a DSSC device using energy relay dyes, tetra-tert-butyl zinc(II) phthalocyanine has been dissolved in the electrolyte of a ruthenium polypyridine complex acceptor, unbound with no direct chemical bond or covalent linker, achieving four-fold increase in the quantum yield for red photons in dye-sensitized nanowire array solar cells.94 The close packed nanoarray architecture was proven to be essential to ensure special Forster Resonance Energy Transfer (FRET) between the donor and the acceptors. In an effort to increase panchromatic response, which is essential to enhance the light harvesting capability thereby solar conversion efficiency, recently multiple energy relay dyes (with complementary absorption characteristics) in the electrolyte has been demonstrated. Two dyes DCM and Rhodamine B with complementary absorption have been used with Dye 15 and high excitation transfer efficiency with 35% increase in the photovoltaic performance has been achieved. Fluorescence resonance energy transfer from both relay dyes to the sensitizing dye was found to be the dominant mechanism for increased light harvesting system; however, the need to design energy relay dyes which are more inert to quenching by the electrolyte was also highlighted.95
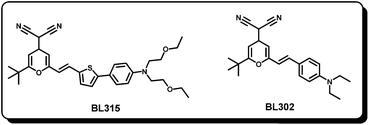
Very recently, two energy relay dyes BL302 and BL315 have been used together with Dye 15 with a 65% increase in the efficiency of the DSSC device (Fig. 17).96 The excitation transfer efficiency was limited to 70% only, which is low compared to the previously reported energy relay dyes. The majority of the losses in excitation transfer efficiency were found to be due to energy transfer to the deabsorbed sensitizing dyes and static quenching of the energy relay dye by complex formation. These studies further suggested that the energy relay dyes have fundamentally different function mechanism and design rules than the sensitizing dyes; this architecture greatly expands the range of dyes that can be used in DSSCs. Still better understanding of the energy relay dye design rules is anticipated to use them for complimentary light harvesting systems in record devices.
10. Conclusions
Considering their optical and electrochemical properties, phthalocyanines have been successfully tested in various practical DSSC applications. In the recent years, the potential of phthalocyanines in dye-sensitized solar cells has been widely explored. Various design strategies yielded stable, near-infrared absorbing, less aggregating phthalocyanines with high internal quantum efficiencies and moreover the directionality of the electron flow in the excited state has also been improved. However, new molecular design strategies for the construction of highly efficient devices are still essential and a profound understanding of the morphology of phthalocyanine sensitizers is indeed necessary in order to control their photophysical properties. On the other hand, the use of energy relay dyes has emerged as a new concept for increasing the light harvesting using these sensitizers through FRET in dye-sensitized solar cells. Though the energy relay dyes have paved the way to new Pc-based cocktail systems for DSSCs, still greater understanding of the energy loss mechanisms in such systems is required.Acknowledgements
VKS thanks to CSIR for a senior research fellowship. LG thanks CSIR-NISE and Department of Science & Technology (DST), Government of India for financial support to carry out this work under two major projects DST-EU (‘ESCORT’) and DST-UK (‘APEX’) and also CSIR-TAPSUN programme.Notes and references
- D. M. Chapin, C. S. Fuller and G. L. Pearson, J. Appl. Phys., 1954, 25, 676–677 CrossRef CAS PubMed.
- K. L. Chopra, P. D. Paulson and V. Dutta, Progr. Photovolt.: Res. Appl., 2004, 12, 69–92 CrossRef CAS.
- M. Gratzel, J. Photochem. Photobiol., C, 2003, 4, 145–153 CrossRef CAS.
- O. Shevaleevskiy, Pure Appl. Chem., 2008, 80, 2079–2089 CrossRef CAS.
- B. O'Regan and M. Grätzel, Nature, 1991, 353, 737–740 CrossRef CAS.
- A. Yella, H. Lee, H. N. Tsao, C. Yi, A. K. Chandiran, M. K. Nazeeruddin, E. W. Diau, C. Y. Yeh, S. M. Zakeeruddin and M. Gratzel, Science, 2011, 334, 629–634 CrossRef CAS PubMed.
- M. K. Nazeeruddin, A. Kay, I. Rodicio, R. Humphry-Baker, E. Muller, P. Liska, N. Vlachopoulos and M. Gratzel, J. Am. Chem. Soc., 1993, 115, 6382–6390 CrossRef CAS.
- P. Wang, S. M. Zakeeruddin, J. E. Moser, M. K. Nazeeruddin, T. Sekiguchi and M. Grätzel, Nat. Mater., 2003, 2, 402–407 CrossRef CAS PubMed.
- T. Bessho, E. Yoneda, J.-H. Hum, M. Guglielmi, I. Tavernelli, H. Imai, U. Rohlisberger, M. K. Nazeeruddin and M. Gratzel, J. Am. Chem. Soc., 2009, 131, 5930–5934 CrossRef CAS PubMed.
- L. Giribabu, C. V. Kumar, Ch. S. Rao, V. G. Reddy, P. Y. Reddy, M. Chadrasekharam and Y. Soujanya, Energy Environ. Sci., 2009, 2, 770–773 CAS.
- L. Giribabu, T. Bessho, M. Srinivasu, C. V. Kumar, Y. Soujanya, V. G. Reddy, P. Y. Reddy, J.-H. Yum, M. Gratzel and M. K. Nazeeruddin, Dalton Trans., 2011, 40, 4497–4504 RSC.
- T. Suresh, G. Rajkumar, S. P. Singh, P. Y. Reddy, A. Islam, L. Y. Han and M. Chandrasekharam, Org. Electron., 2013, 14, 2243–2248 CrossRef CAS PubMed.
- S. Ito, H. Miura, S. Uchida, M. Takata, K. Sumioka, P. Liska, P. Comte, P. Pechy and M. Gratzel, Chem. Commun., 2008, 5194–5196 RSC.
- K. Hara, Z.-S. Wang, T. Sato, A. Furube, R. Katoh, H. Sugihara, Y. Dan-oh, C. Kasada, A. Shinpo and S. Suga, J. Phys. Chem. B, 2005, 109, 15476–15482 CrossRef CAS PubMed.
- R. Chen, X. Yang, H. Tian and L. Sun, J. Photochem. Photobiol., A, 2007, 189, 295–300 CrossRef CAS PubMed.
- G. Zhang, H. Bala, Y. Cheng, D. Shi, X. Lv, Q. Yu and P. Wang, Chem. Commun., 2009, 2198–2200 RSC.
- R. K. Kanaparthi, J. Kandhadi and L. Giribabu, Tetrahedron, 2012, 68, 8383–8393 CrossRef CAS PubMed.
- L. Giribabu, R. K. Kanaparthi and V. Velkannan, Chem. Rec., 2012, 12, 306–328 CrossRef CAS PubMed.
- L. Giribabu and R. K. Kanaparthi, Curr. Sci., 2013, 104, 847–855 CAS.
- L. Giribabu, K. Sudhakar and V. Velkannan, Curr. Sci., 2012, 102, 991–1000 CAS.
- H. Imahori, T. Umeyama and S. Ito, Acc. Chem. Res., 2009, 42, 1809–1818 CrossRef CAS PubMed.
- I. Radivojevic, A. Varotto, C. Farley and C. M. Drain, Energy Environ. Sci., 2010, 3, 1897–1909 CAS.
- M. V. Martinez Diaz, G. de la Torre and T. Torres, Chem. Commun., 2010, 46, 7090–7108 RSC.
- M. G. Walter, A. B. Rudine and C. C. Wamser, J. Porphyrins Phthalocyanines, 2010, 14, 759–792 CrossRef CAS.
- M. J. Griffith, K. Sunahara, P. Wagner, K. Wagner, G. G. Wallace, D. L. Officer, A. Furube, R. Katoh, S. Mori and A. J. Mozer, Chem. Commun., 2012, 48, 4145–4162 RSC.
- H. Imahori, T. Umeyama, K. Kurotobi and Y. Takano, Chem. Commun., 2012, 48, 4032–4045 RSC.
- M. K. Panda, K. Ladomenou and A. G. Coutsolelos, Coord. Chem. Rev., 2012, 256, 2601–2627 CrossRef CAS PubMed.
- M. V. Martinez Diaz, M. Ince and T. Torres, Monatsh. Chem., 2011, 142, 699–707 CrossRef CAS.
- B. O'Regan and M. Gratzel, Nature, 1991, 353, 737–740 CrossRef CAS.
- M. K. Nazeeruddin, A. Kay, I. Rodicio, R. Humphry-Baker, E. Muller, P. Liska, N. Vlachopoulos and M. Gratzel, J. Am. Chem. Soc., 1993, 115, 6382–6390 CrossRef CAS.
- A. Hagfeldt, G. Boschloo, L. C. Sun, L. Kloo and H. Pettersson, Chem. Rev., 2010, 110, 6595–6663 CrossRef CAS PubMed.
- L. Valli, Adv. Colloid Interface Sci., 2005, 116, 13–44 CrossRef CAS PubMed.
- A. Pailleret, F. Bedioui, in N4-Macrocyclic Metal Complexes, ed. J. Zagal, F. Bedioui and J. P. Dodelet, Springer, New York, 2006, pp. 363–423, ch. 8 Search PubMed.
- C. G. Classens, W. J. Blau, M. Cook, M. Hanack, R. J. M. Nolte, T. Torres and D. Wöhrle, Monatsh. Chem., 2001, 132, 3–11 CrossRef.
- A. J. Duro, G. de la Torre, J. Barber, J. L. Serrano and T. Torres, Chem. Mater., 1996, 8, 1061–1066 CrossRef.
- P. S. Lai, P. J. Lou, C. L. Peng, C. L. Pai, W. N. Yen, M. Y. Huang, T. H. Young and M. J. Shieh, J. Controlled Release, 2007, 122, 39–46 CrossRef CAS PubMed.
- S. V. Rao, N. Venkatram, L. Giribabu and D. N. Rao, J. Appl. Phys., 2009, 105, 053109 CrossRef PubMed.
- M. K. Nazeeruddin, R. H. Baker, M. Grätzel, D. Wöhrle, G. Schnurpfeil, G. Schneider, A. Hirth and N. Trombach, J. Porphyrins Phthalocyanines, 1999, 3, 230–237 CrossRef CAS.
- V. Aranyos, J. Hjelm, A. Hagfeldt and H. Grennberg, J. Porphyrins Phthalocyanines, 2001, 5, 609–616 CrossRef CAS.
- L. Yong, L. Peifen, Y. Xingzhong, J. Lu and P. Zhonghua, RSC Adv., 2013, 3, 545–558 RSC.
- J. He, G. Benkö, F. Korodi, T. Polívka, R. Lomoth, B. Akermark, L. Sun, A. Hagfeldt and V. Sundström, J. Am. Chem. Soc., 2002, 124, 4922–4932 CrossRef CAS PubMed.
- Y. Amao and T. Komori, Langmuir, 2003, 19, 8872–8875 CrossRef CAS.
- T. Komori and Y. Amao, J. Porphyrins Phthalocyanines, 2003, 7, 131–136 CrossRef CAS.
- P. Balaraju, M. Kumar, M. S. Roy and G. D. Sharma, Synth. Met., 2009, 159, 1325–1331 CrossRef PubMed.
- L. Jin, Z. L. Ding and D. J. Chen, J. Mater. Sci., 2013, 48, 4883–4891 CrossRef CAS.
- M. K. Nazeeruddin, R. Humphry-Baker, M. Grätzel and B. A. Murrer, Chem. Commun., 1998, 719 RSC.
- M. Yanagisawa, F. Korodi, J. He, L. Su, V. Sundstrom and B. Akermark, J. Porphyrins Phthalocyanines, 2002, 6, 217–224 CrossRef CAS.
- A. Morandeira, I. Lopez-Duarte, B. O’Regan, M. V. Martinez-Diaz, A. Forneli, E. Palomares, T. Torres and J. R. Durrant, J. Mater. Chem., 2009, 19, 5016–5026 RSC.
- E. Palomares, M. V. Martinez Diaz, S. A. Haque, T. Torres and J. R. Durrant, Chem. Commun., 2004, 2112–2113 RSC.
- B. Lim, G. Y. Margulis, J. Yum, E. L. Unger, B. E. Hardin, M. Gratzel, M. D. McGehee and A. Sellinger, Org. Lett., 2013, 15, 784–787 CrossRef CAS PubMed.
- M. Yanagisawa, F. Korodi, J. Bergquist, A. Holmberg, A. Hagfeldt, B. Akermark and L. Sun, J. Porphyrins Phthalocyanines, 2004, 8, 1228–1235 CrossRef CAS.
- P. Y. Reddy, L. Giribabu, C. Lyness, H. J. Snaith, Ch. Vijaykumar, M. Chandrasekharam, M. Lakshmikantam, J. H. Yum, K. Kalyanasundaram, M. Grätzel and M. K. Nazeeruddin, Angew. Chem., Int. Ed., 2007, 46, 373–376 CrossRef CAS PubMed.
- L. Giribabu, Ch. Vijay Kumar, V. Gopal Reddy, P. Yella Reddy, Ch. Srinivasa Rao, S.-R. Jang, J.-H. Yum, Md. K. Nazeeruddin and M. Grätzel, Sol. Energy Mater. Sol. Cells, 2007, 91, 1611–1617 CrossRef CAS PubMed.
- L. Giribabu, C. V. Kumar, M. Raghavender, K. Somaiah, P. Y. Reddy and P. Y. Rao, J. Nano Res., 2008, 2, 39–48 CrossRef CAS.
- J. J. Cid, J. H. Yum, S. R. Jang, M. K. Nazeeruddin, E. M. Ferrero, E. Palomares, J. Ko, M. Grätzel and T. Torres, Angew. Chem., Int. Ed., 2007, 46, 8358–8362 CrossRef CAS PubMed.
- L. Giribabu, C. Vijaykumar, P. Y. Reddy, J. H. Yum, M. Grätzel and M. K. Nazeeruddin, J. Chem. Sci., 2009, 121, 75–82 CrossRef CAS PubMed.
- J. J. Cid, M. G. Iglesias, J. H. Yum, A. Forneli, J. Albero, E. M. Ferrero, P. Vázquez, M. Grätzel, M. K. Nazeeruddin, E. Palomares and T. Torres, Chem.–Eur. J., 2009, 15, 5130–5137 CrossRef CAS PubMed.
- M. G. Iglesias, J. J. Cid, J. H. Yum, A. Forneli, P. Vázquez, M. K. Nazeeruddin, E. Palomares, M. Grätzel and T. Torres, Energy Environ. Sci., 2011, 4, 189–194 Search PubMed.
- M. Garcia-Iglesias, J. Yum, R. H. Baker, S. M. Zakeeruddin, P. Pechy, P. Vazquez, E. Palomares, M. Gratzel, M. K. Nazeeruddin and T. Torres, Chem. Sci., 2011, 2, 1145–1150 RSC.
- M. Ragoussi, J. J. Cid, J. Yum, G. Torre, D. D. Censo, M. Gratzel, M. K. Nazeeruddin and T. Torres, Angew. Chem., Int. Ed., 2012, 51, 4375–4378 CrossRef CAS PubMed.
- I. Lopez-Duarte, M. Wang, R. H. Baker, M. Ince, M. Victoria Martinez-Diaz, M. K. Nazeeruddin, T. Torres and M. Gratzel, Angew. Chem., Int. Ed., 2012, 51, 1895–1898 CrossRef CAS PubMed.
- L. Giribabu, V. K. Singh, C. Vijaykumar, Y. Soujanya, P. Y. Reddy and M. L. Kantam, Sol. Energy, 2011, 85, 1204–1212 CrossRef CAS PubMed.
- M. Ince, F. Cardinali, J. Yum, M. V. Martinez-Diaz, M. K. Nazeeruddin, M. Gratzel and T. Torres, Chem.–Eur. J., 2012, 18, 6343–6348 CrossRef CAS PubMed.
- M. Durmus and T. Nyokong, Polyhedron, 2007, 26, 2767–2776 CrossRef CAS PubMed.
- N. B. McKeown, S. Makheed, K. J. Msayib, L. L. Ooi, M. Hellowell and J. E. Wareen, Angew. Chem., Int. Ed., 2005, 44, 7546 CrossRef CAS PubMed.
- S. Eu, T. Katoh, T. Umeyama, Y. Matano and H. Imahori, Dalton Trans., 2008, 5476–5483 RSC.
- S. Mori, M. Nagata, Y. Nakahata, K. Yasuta, R. Goto, M. Kimura and M. Taya, J. Am. Chem. Soc., 2010, 132, 4054–4055 CrossRef CAS PubMed.
- E. M. Barea, J. Ortiz, F. J. Paya, F. F. Lazaro, F. Fabregat-Santiago, A. Sastre-Santos and J. Bisquert, Energy Environ. Sci., 2010, 3, 1985–1994 CAS.
- M. Kimura, H. Nomoto, N. Masaki and S. Mori, Angew. Chem., 2012, 124, 4447 (Angew. Chem., Int. Ed., 2012, 51, 4371) CrossRef.
- M. Kimura, H. Nomoto, H. Suzuki, T. Ikeuchi, H. Matsuzaki, T. N. Murakami, A. Furube, N. Masaki, M. J. Griffith and S. Mori, Chem.–Eur. J., 2013, 19, 7496–7502 CrossRef CAS PubMed.
- L. Giribabu, V. K. Singh, T. Jella, Y. Soujanya, A. Amat, F. De Angelis, A. Yella, P. Gao and M. K. Nazeeruddin, Dyes Pigm., 2013, 98, 518–529 CrossRef CAS PubMed.
- V. K. Singh, P. Salvatori, A. Amat, S. Agrawal, F. De Angelis, M. K. Nazeeruddin, N. V. Krishna and L. Giribabu, Inorg. Chim. Acta, 2013, 407, 289–296 CrossRef CAS PubMed.
- L. Y. Han, A. Islam, H. Chen, C. Malapaka, B. Chiranjeevi, S. F. Zhang, X. D. Yang and M. Yanagida, Energy Environ. Sci., 2012, 5, 6057–6060 CAS.
- R. Y. Ogura, S. Nakane, M. Morooka, M. Orihashi, Y. Suzuki and K. Noda, Appl. Phys. Lett., 2009, 94, 073308 CrossRef PubMed.
- Y. Chen, Z. Zeng, C. Li, W. Wang, X. Wang and B. Zhang, New J. Chem., 2005, 29, 773–776 RSC.
- D. Kuang, P. Walter, F. Nuesch, S. Kim, J. Ko, P. Comte, S. M. Zakeeruddin, M. K. Nazeeruddin and M. Gratzel, Langmuir, 2007, 23, 10906–10909 CrossRef CAS PubMed.
- T. Bessho, S. M. Zakeeruddin, C. Y. Yeh, E. W. G. Diau and M. Gratzel, Angew. Chem., Int. Ed., 2010, 49, 6646–6649 CrossRef CAS PubMed.
- M. S. Kang, S. H. Kang, S. G. Kim, I. T. Choi, J. H. Ryu, M. J. Ju, D. Cho, J. Y. Lee and H. K. Kim, Chem. Commun., 2012, 48, 9349–9351 RSC.
- J. H. Yum, S. R. Jang, P. Walter, T. Geiger, F. Nuesch, S. Kim, J. Ko, M. Gratzel and M. K. Nazeeruddin, Chem. Commun., 2007, 4680–4682 RSC.
- S. Kim, J. K. Lee, S. O. Kang, J. Ko, J. H. Yum, S. Fantacci, F. De Angelis, D. Di Censo, M. K. Nazeeruddin and M. Gratzel, J. Am. Chem. Soc., 2006, 128, 16701–16707 CrossRef CAS PubMed.
- L. S. Mende, U. Bach, R. H. Baker, T. Horiuchi, H. Miura, S. Ito and M. Gratzel, Adv. Mater., 2005, 17, 813–815 CrossRef.
- W. H. Howie, F. Claeyssens, H. Miuraand and L. Perter, J. Am. Chem. Soc., 2008, 130, 1367–1375 CrossRef CAS PubMed.
- T. Dentani, Y. Kubota, K. Furnabiki, J. Jin, T. Yoshida, H. Minoura, H. Miura and M. Matsui, New J. Chem., 2009, 33, 93–101 RSC.
- J. N. Clifford, E. Palomares, M. K. Nazeeruddin, M. Gratzel and J. R. Durrant, J. Phys. Chem. C, 2007, 111, 6561–6567 CAS.
- M. K. Nazeeruddin, A. Kay, I. Rodicio, R. Humphry-Baker, E. Muller, P. Liska, N. Vlachopoulos and M. Gratzel, J. Am. Chem. Soc., 1993, 115, 6382–6390 CrossRef CAS.
- Y. Tachibana, J.-E. Moser, M. Gratzel, D. R. Klug and J. R. Durrant, J. Phys. Chem., 1996, 100, 20056–20062 CrossRef CAS.
- S. A. Haque, Y. Tachibana, R. L. Willis, J.-E. Moser, M. Gratzel, D. R. Klug and J. R. Durrant, J. Phys. Chem. B, 2000, 104, 538–547 CrossRef CAS.
- Y. Tachibana, M. K. Nazeeruddin, M. Gratzel, D. R. Klug and J. R. Durrant, Chem. Phys., 2002, 285, 127–132 CrossRef CAS.
- B. E. Hardin, E. T. Hoke, C. Peters, T. Geiger, F. Nuesch, M. Gratzel, M. K. Nazeeruddin and M. D. McGehee, in Long Range Forster Energy Transfer to Increase Light Absorption in Dye-Sensitized Solar Cells Materials Research Society Fall Meeting, Materials Research Society, Boston, MA, 2008 Search PubMed.
- J. H. Yum, B. E. Hardin, S. J. Moon, E. Baranoff, F. Nuesch, M. D. McGehee, M. Gratzel and M. K. Nazeeruddin, Angew. Chem., Int. Ed., 2009, 48, 9277–9280 CrossRef CAS PubMed.
- E. T. Hoke, B. E. Hardin and M. D. McGehee, Opt. Express, 2010, 18(4), 3893–3904 CrossRef CAS PubMed.
- B. E. Hardin, E. T. Hoke, P. B. Armstrong, J. H. Yum, P. Comte, T. Torres, J. M. Frechet, M. K. Nazeeruddin, M. Gratzel and M. D. McGehee, Nat. Photonics, 2009, 3, 406–411 CrossRef CAS.
- B. E. Hardin, J.-H. Yum, E. T. Hoke, Y. C. Jun, P. Pechy, T. Torres, M. L. Brongersma, M. K. Nazeeruddin, M. M. Gratzel and D. McGehee, Nano Lett., 2010, 10, 3077–3083 CrossRef CAS PubMed.
- K. Shankar, X. Feng and C. A. Grimes, ACS Nano, 2009, 3, 788–794 CrossRef CAS PubMed.
- J.-H. Yum, B. E. Hardin, E. T. Hoke, E. Baranoff, S. M. Zaeeruddin, M. K. Nazeeruddin, T. Torres, M. D. McGehee and M. Gratzel, ChemPhysChem, 2011, 12, 657–661 CrossRef CAS PubMed.
- G. Y. Margulis, B. Lim, B. E. Hardin, E. L. Unger, J. H. Yum, J. M. Feckl, D. F. Rohlfing, T. Bein, M. Gratzel, A. Sellinger and M. D. McGehee, Phys. Chem. Chem. Phys., 2013, 15, 11306–11312 RSC.
| This journal is © The Royal Society of Chemistry 2014 |