The preparation of hierarchical tubular structures comprised of NiO nanosheets with enhanced supercapacitive performance
Xin Xua,
Jin Lianga,
Han Zhoua,
Shujiang Ding*ab and
Demei Yu*a
aMOE Key Laboratory for Nonequilibrium Synthesis and Modulation of Condensed Matter and Department of Applied Chemistry, School of Science, Xi'an Jiaotong University, Xi'an 710049, China. E-mail: dingsj@mail.xjtu.edu.cn; dmyu@mail.xjtu.edu.cn
bState Key Laboratory for Mechanical Behaviour of Materials, Xi'an Jiaotong University, Xi'an 710049, China
First published on 2nd December 2013
Abstract
Hierarchically tubular structures comprised NiO nanosheets were successfully prepared through a mild solution route based on the template of polymeric nanotubes (PNT) followed by a thermal annealing treatment. The microstructure and chemical composition of NiO nanosheets nanotubes are investigated by SEM, TEM, HRTEM, SAED and XRD. The Brunauer–Emmett–Teller (BET) specific surface area of this sample is calculated to be 98 m2 g−1 and the majority of pores have a size in the range of 2–10 nm. The thermal behavior of Ni-precursor@PNT was studied by TGA and the weight fraction of NiO nanosheets nanotubes obtained by calcination is measured to be 57.0%. The specific capacitance of the unique NiO nanosheets nanotubes is 588 F g−1 at the end of 1000 cycles when the charge–discharge current density is 3 A g−1, leading to only 5.2% capacity loss. In addition, the NiO nanotubes coating by relatively sparse and thin nanosheets possess better electrochemical properties. The specific capacitance is 960 F g−1 at the end of 1000 cycles when the charge–discharge current density is 10 A g−1, leading to only 1.2% capacity loss. Broadly, the as-obtained NiO nanosheets nanotubes reveal relatively high capacitance and remarkable cycling stability in virtue of the hollow, porous, flaky and tubular nanostructures.
1. Introduction
In recent years, rapidly increasing demands for energy storage of consumer electronic devices and electric vehicles have called for intense research on high performance electrode materials.1 Supercapacitors, with reasonably higher energy and power densities, faster charge–discharge rate and longer working lifetime, have been considered as one pivotal electric energy storage device together with lithium-ion batteries (LIBs).2–5 On account of their intriguing electrochemical properties, supercapacitors can be used for momentary energy smoothing load services such as emergency power supplies, peak power assistance for batteries in electric vehicles and start-up power supply for tank in cold zone.6 For this reason, plenty of materials were investigated as promising electrode materials for supercapacitors as two major types. (i) Pseudocapacitive materials, such as metal hydroxides,6–8 transition metal oxides9 and conducting polymers10,11 have being researched because of their high specific capacitances and high energy densities. However, they usually suffer from fast decay of rate capability and poor cycle stability; (ii) carbon based materials, which possessed large specific surface area, charging–discharging by an electric double layer mechanism. This kind of materials usually exhibited high power density, excellent rate performance and long cycle life, but showed relatively low specific capacitance.4,12–18 In particular, the energy density of pseudocapacitive materials is generally greater than that of carbon based materials, hence, more attention is focused on the former. To overcome the above problems, it is necessary to mention the redox reaction between the active materials and electrolyte ions during the charge–discharge process. It is generally accepted that the ultrathin surface layer between active materials and the electrolyte ions provides place for redox reaction. The contact area between active materials and the electrolyte determine the specific capacitance and rate performance of supercapacitors. Thus, fabricating pseudocapacitive materials with porous nanostructures to acquire larger specific surface area is a valid method.Nickel oxide (NiO), as a kind of transition metal oxide, has been widely studied for energy storage applications, including lithium-ion batteries and supercapacitors in view of its extremely high theoretical specific capacity, and revealed outstanding electrochemical performance compared to the traditional materials.19–29 In the recent work by Liang et al., they prepared NiO film on the high-purity copper sheet and these nanoporous NiO displayed a high specific capacity of 1790 F g−1 at a discharge current density of 2.5 A g−1 due to a extremely close contact between active materials and conductive substrate.30 Nevertheless, the capacitance of the existing NiO electrode materials prepared by facile chemical method are still far below its theoretical value (2584 F g−1), and the cycling performance is also not desirable. In consideration of the strategy mentioned above, constructing hierarchical porous and hollow structured NiO via a facile method is meaningful to enhance the supercapacitive performance. In this work, we use polymeric nanotubes (PNTs) with uniform size as hard templates for preparation of NiO nanosheets nanotubes, which is synthesized by cationic polymerization of divinylbenzene using immiscible initiator nanodroplets of boron trifluoride etherate complex31 and followed a sulfonation process. These uniform PNTs have three main advantages as templates: (i) the positively charged precursor ions can be easily adsorbed to the surface of the sulfonated PNTs due to the electrostatic interaction with the negative functional groups (–SO3); (ii) PNTs can be easily removed through calcination at a lower temperature, by which the nanostructure of metal oxide can be well preserved; (iii) the produced gas and osmotic pressure during the calcination can be released through the inner channels and open ends of the PNTs.32,33
Herein, we report the synthesis of NiO nanosheets nanotubes by a simple and economical solution route. The synthetic process is illustrated in Scheme 1. Firstly, we prefabricate Ni-precursor@PNT based on in situ growth of Ni2CO3(OH)2 nanosheets from the sulfonated gel matrix of polymeric nanotubes. After being calcined in air, the well-developed mesoporous NiO nanosheets nanotubes are successfully obtained. The as-prepared NiO nanosheets nanotubes are believed to be very suitable as the electrode material for supercapacitors for at least three reasons: firstly, NiO nanosheets nanotubes with higher specific surface area can provide more sites for redox reaction between the active materials and electrolyte ions, leading to higher rate performance and specific capacity. Secondly, massive void spaces derived from cavity and piling up nanosheets could effectively buffer the strain generated during the fast charging–discharging process, hence improving the cycling performance. Lastly, the two ends of the nanotubes are totally open, providing additional paths for ions in solution.
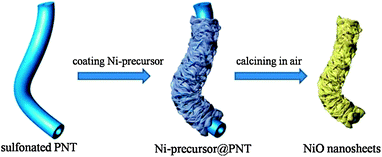 | ||
| Scheme 1 Schematic illustration of the synthetic procedure of NiO nanosheets nanotubes hierarchical structures. | ||
2. Experimental
2.1. Material synthesis
Sulfonated PNTs: PNTs were prepared according to a previously reported method.31 PNTs (3 g) were added to concentrated sulfuric acid (PNTs![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2SO4 = 1
H2SO4 = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30, w/w) and the mixture was ultrasonicated for 10 min to ensure well dispersion. After being stirred for 24 h at 40 °C, the red precipitate was collected by centrifugation and washed thoroughly with ethanol.
30, w/w) and the mixture was ultrasonicated for 10 min to ensure well dispersion. After being stirred for 24 h at 40 °C, the red precipitate was collected by centrifugation and washed thoroughly with ethanol.
Ni-precursor@PNT: 75 mg Ni(NO3)2·6H2O and 35 mg hexamethylenetetramine (HMT) were dissolved into 40 mL 0.7 mM trisodium citrate solution. Then 5 mg sulfonated PNTs were dispersed into the above solution by sonication for 10 minutes. The reaction mixture was transferred into a sealed bottle and kept in an electric oven at 90 °C for 6 h. The bottle was then taken out of the oven and left to cool down to room temperature. The green precipitate was collected by centrifugation, washed thoroughly with ethanol, and dried at 60 °C overnight.
NiO nanosheets nanotubes: To obtain the NiO nanosheets nanotubes, the as-prepared Ni-precursor@PNT composite was subjected to calcination at 450 °C for 2 h to remove the PNTs templates and obtain the black product.
The NiO nanotubes coating by relatively sparse and thin nanosheets were prepared by the same conditions with a slightly magnetic stirring.
2.2. Characterization
The product morphology was examined using field-emission scanning electron microscopy (FESEM; HITACHI, su-8010) and transmission electron microscopy (TEM; JEOL, JEM-2100). Crystallographic information of the samples was collected using powder X-ray diffraction (XRD; SHIMADZU, Lab X XRD-6000). Thermogravimetric analysis (Perkin-Elmer TGA 7) was carried out under a flow of air with a temperature ramp of 10 °C min−1 from room temperature to 600 °C. The specific surface area and pore size distribution of the products were measured using a BET analyzer (ASAP 2020M) at 77 K.2.3. Electrochemical measurements
The working electrode was prepared by mixing 70 wt% of the active material (NiO nanosheets nanotubes), 20 wt% of conducting agent (carbon black, super-P-Li), and 10 wt% of binder (polyvinylidene difluoride, PVDF, Aldrich). This mixture was then pressed onto the glassy carbon electrode (Aida Hengsheng Technology co. Ltd, Tianjn, China) and dried at 60 °C. The electrolyte used was a 2 M KOH aqueous solution. The capacitive performance of the samples was tested on a CHI 660D electrochemical workstation with cyclic voltammetry and chronopotentiometry functions using a three-electrode cell where Pt foil serves as the counter electrode and a standard calomel electrode (SCE) as the reference electrode.3. Results and discussion
Fig. 1 shows the SEM and TEM images of the Ni-precursor@PNT. As can be seen, almost every PNT is covered with Ni-precursor nanosheets, this may be attributed to the large amount of functional groups (–SO3) uniformly distributed on the surface of the PNTs. The Ni-precursor@PNT displays tubular and flaky nanostructure with diameter of around 500–600 nm (Fig. 1A). In order to well observe the internal structure of the Ni-precursor@PNT, we subsequently apply the powerful ultrasonic treatment to peel off partial Ni-precursor nanosheets. From Fig. 1B, it can be clearly observed that the internal diameter of the Ni-precursor@PNT is approximately 200 nm, which is consistent with the outer diameter of the PNTs. It can be seen distinctly from the TEM image of Ni-precursor@PNT that the PNTs nanotubes with average diameter of 200 nm are uniformly assembled by Ni-precursor nanoclusters that consist of ultrathin nanosheets (Fig. 1C). A further TEM observation reveal the hierarchical structure of the Ni-precursor@PNT (Fig. 1D).The microstructure and chemical composition of NiO nanosheets nanotubes are investigated by SEM, TEM, HRTEM and SAED with the results shown in Fig. 2. Fig. 2A and B show the SEM images of NiO nanosheets nanotubes obtained after calcining the Ni-precursor@PNT composite at 450 °C for 2 h. The diameter of the hierarchically structured NiO nanosheets nanotubes is about 450–550 nm, smaller than the Ni-precursor@PNT, possibly because of the partial collapse of the NiO nanosheets nanotubes hollow structure at the elevated temperature. The calcined NiO nanosheets nanotubes product maintains its tubular and sheets-like structures after removing the PNT templates, which is apparent that the NiO nanosheets nanotubes show excellent thermal stability (Fig. 2A). Moreover, it is very clear from Fig. 2B that the end of the nanotube is totally open and the internal diameter decreases to about 180 nm which is smaller than the outer diameter of PNTs, owing to the removing of PNTs templates. The open ends of the NiO nanostructures enhance the accessibility by the electrolyte solution which is beneficial to the electrochemical performance of NiO nanosheets nanotubes composite. Fig. 2C and D display that the NiO nanosheets nanotubes sample has an extra porous structure derived from piling up nanosheets, and the thickness of the NiO nanosheets is approximately 10–15 nm. The HRTEM image (Fig. 2E) taken from the NiO nanosheets show interplanar spacing of 0.21 nm, corresponding to that for the (200) facet of face-centered cubic phase NiO. The selected-area electron diffraction (SAED) pattern (Fig. 2F) shows five intense rings indexed to the (111), (200), (220), (311), (222) planes of NiO nanosheets nanotubes. The as-prepared NiO nanosheets nanotubes possess porous characteristic and large specific surface area, with the test results shown in Fig. 3. Nitrogen adsorption/desorption isotherm of the NiO nanosheets nanotubes is shown in Fig. 3A, and the corresponding Barrett–Joyner–Halenda (BJH) pore size distribution obtained from the desorption branch of the isotherm is shown in Fig. 3B. The Brunauer–Emmett–Teller (BET) specific surface area of this sample is calculated to be 98 m2 g−1. It can also be observed that the majority of pores have a size in the range of 2–10 nm.
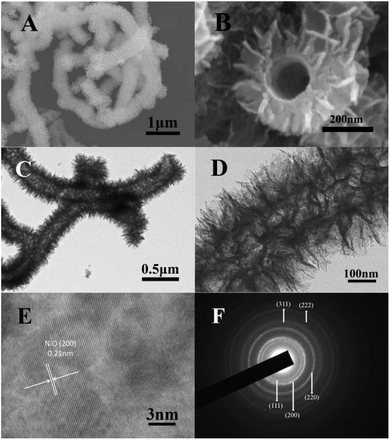 | ||
| Fig. 2 SEM (A and B) images, TEM (C and D) images, HRTEM image (E) and SAED pattern (F) of NiO nanosheets nanotubes hierarchical structures. | ||
 | ||
| Fig. 3 (A) Nitrogen adsorption/desorption isotherm of the NiO nanosheets nanotubes. (B) The pore size distribution calculated using the BJH method from the desorption curve. | ||
Fig. 4A shows the corresponding XRD patterns of the Ni-precursor@PNT and NiO nanosheets nanotubes. The XRD pattern of the Ni-precursor can be assigned to monoclinic Ni2CO3(OH)2 (JCPDS file no. 35-0501, space group: P21/*, a0 = 9.236 Å, b0 = 12.001 Å and c0 = 3.091 Å), while that of the annealed sample can be unambiguously assigned to face-centered cubic phase of NiO (JCPDS file no. 4-835, space group: Fm3m, a0 = 4.1769 Å), which is consistent with the SAED results.
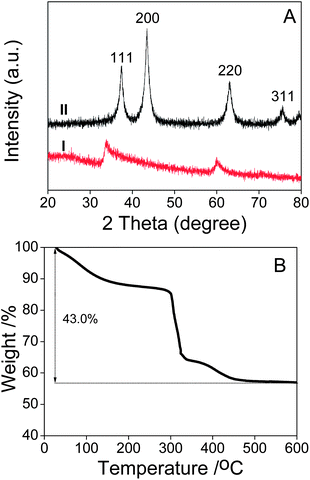 | ||
| Fig. 4 (A) XRD patterns of Ni-precursor@PNT composite (I) and NiO nanosheets nanotubes (II); (B) TGA curve of the Ni2CO3(OH)2@PNT composite. | ||
The thermal behavior of Ni-precursor@PNT was studied by TGA, with the results shown in Fig. 4B. The initial weight loss of Ni-precursor@PNT below 320 °C can be mainly attributed to the removal of physically adsorbed water and partial decomposition of Ni2CO3(OH)2. The Ni-precursor@PNT shows significant weight loss at about 400 °C, which can be mainly attributed to the decomposition of the sulfonated PNTs templates.31 Finally, the curve flattens out at approximately 450 °C, indicating that the PNTs templates are removed and the Ni-precursor is converted to NiO. After reaching 600 °C, the Ni-precursor@PNT shows a total weight loss of 43.0%, and the weight fraction of NiO nanosheets obtained by calcination is measured to be 57.0%.
To prove the superiority of the hierarchical NiO nanosheets nanotubes, we subsequently investigate the electrochemical properties of the sample as an electrode for super-capacitors. Fig. 5A shows the cyclic voltammetry (CV) curves of the as-obtained NiO nanosheets nanotubes during the anodic and cathodic sweeps, which is owing to the reversible reduction as described by reaction (1):
| NiO + OH− ↔ NiOOH + e− | (1) |
| Cm = I × Δt/(ΔV × m) | (2) |
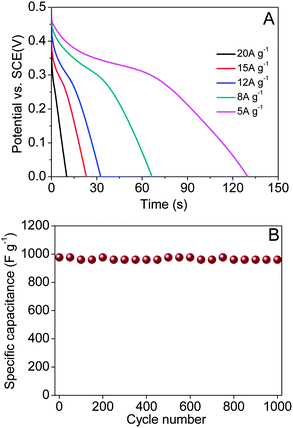
Furthermore, we ameliorate the morphology of the NiO nanosheets with a slightly magnetic stirring in the same synthesis conditions, the as-obtained NiO nanosheets coating on the NiO nanotubes turn sparse and thin (Fig. 6). The disturbance of the reaction system make less Ni-precursor be adsorbed to the PNTs surface, and the nanosheets may become thinner during their process of growth. We subsequently investigate the electrochemical properties of these NiO nanosheets nanotubes as an electrode for super-capacitors. As can be seen in Fig. 7A, the specific capacitance is as high as 1299, 1064, 780, 690 and 404 F g−1 at the current densities of 5, 8, 12, 15 and 20 A g−1, respectively. Moreover, the cycling performance of the newly prepared NiO nanosheets is more stable. When the charge–discharge current density is 10 A g−1, the specific capacitance of the new NiO nanosheets is 960 F g−1 at the end of 1000 cycles, leading to only 1.2% capacity loss. These facts proved that these sparse and thin NiO nanosheets coating on the NiO nanotubes possess better electrochemical properties.
 | ||
| Fig. 6 SEM (A) images, TEM (C and D) images of the Ni-precursor@PNT; SEM (A) images, TEM (C and D) images of the NiO nanosheets nanotubes hierarchical structures. | ||
4. Conclusions
In summary, we have developed a facile method to produce NiO nanosheets nanotubes using sulfonated polymeric nanotubes as templates. The unique NiO nanosheets nanotubes possess porous, flaky and hollow structures, thus the products can offer more contact area between active materials and the electrolyte ions, buffer the large volume change during the fast charging–discharging process simultaneously. Furthermore, the entirely open ends of the NiO nanosheets nanotubes provide additional paths for electrolyte ions. These advantages could enhance the electrochemical performance of the NiO nanosheets nanotubes as an electrode material for supercapacitors. Naturally, it revealed that these superior NiO nanomaterials manifest relatively high capacitance and remarkable cycling stability compared to other electrode materials for supercapacitors. This method could also be used to prepare other metal oxide nanomaterials such as Co3O4, NiCo2O4. These related works are in progress.Acknowledgements
This research was supported partially by the National Natural Science Foundation of China (no. 51273158, 21303131); Natural Science Basis Research Plan in Shaanxi Province of China (no. 2012JQ6003, 2013KJXX-49); Ph.D. Programs Foundation of Ministry of Education of China (no. 20120201120048); Program for New Century Excellent Talents in University (NCET-13-0449). The authors are grateful to the Fundamental Research Funds for the Central Universities for financial support.References
- A. S. Arico, P. Bruce, B. Scrosati, J. M. Tarascon and W. Van Schalkwijk, Nat. Mater., 2005, 4, 366–377 CrossRef CAS PubMed.
- J. R. Miller and P. Simon, Science, 2008, 321, 651–652 CrossRef CAS PubMed.
- C. Peng, S. W. Zhang, X. H. Zhou and G. Z. Chen, Energy Environ. Sci., 2010, 3, 1499–1502 Search PubMed.
- L. L. Zhang and X. S. Zhao, Chem. Soc. Rev., 2009, 38, 2520–2531 RSC.
- B. Wang, J. S. Chen, Z. Y. Wang, S. Madhavi and X. W. Lou, Adv. Energy Mater., 2012, 2, 1188–1192 CrossRef CAS.
- H. L. Wang, H. S. Casalongue, Y. Y. Liang and H. J. Dai, J. Am. Chem. Soc., 2010, 132, 7472–7477 CrossRef CAS PubMed.
- S. Nohara, A. A. Toshihide, H. Wada, N. Furukawa, H. Inoue, N. Sugoh, H. Iwasaki and C. Iwakura, J. Power Sources, 2006, 157, 605–609 CrossRef CAS PubMed.
- J. H. Park, O. O. Park, K. H. Shin, C. S. Jin and J. H. Kim, Electrochem. Solid-State Lett., 2002, 5, H7–H10 CrossRef CAS PubMed.
- S. C. Pang, M. A. Anderson and T. W. Chapman, J. Electrochem. Soc., 2000, 147, 444–450 CrossRef CAS PubMed.
- Y. G. Wang, H. Q. Li and Y. Y. Xia, Adv. Mater., 2006, 18, 2619 CrossRef CAS.
- K. Zhang, L. L. Zhang, X. S. Zhao and J. S. Wu, Chem. Mater., 2010, 22, 1392–1401 CrossRef CAS.
- P. Sharma and T. S. Bhatti, Energy Convers. Manage., 2010, 51, 2901–2912 CrossRef CAS PubMed.
- H. M. Sun, L. Y. Cao and L. H. Lu, Energy Environ. Sci., 2012, 5, 6206–6213 CAS.
- J. T. Zhang, J. W. Jiang, H. L. Li and X. S. Zhao, Energy Environ. Sci., 2011, 4, 4009–4015 CAS.
- C. X. Guo and C. M. Li, Energy Environ. Sci., 2011, 4, 4504–4507 CAS.
- Y. Kou, Y. H. Xu, Z. Q. Guo and D. L. Jiang, Angew. Chem., Int. Ed., 2011, 50, 8753–8757 CrossRef CAS PubMed.
- S. W. Lee, B. S. Kim, S. Chen, Y. Shao-Horn and P. T. Hammond, J. Am. Chem. Soc., 2009, 131, 671–679 CrossRef CAS PubMed.
- D. W. Wang, F. Li, M. Liu, G. Q. Lu and H. M. Cheng, Angew. Chem., Int. Ed., 2008, 47, 373–376 CrossRef CAS PubMed.
- B. Wang, J. S. Chen, Z. Y. Wang, S. Madhavi and X. W. Lou, Adv. Energy Mater., 2012, 2, 1188–1192 CrossRef CAS.
- F. Cao, F. Zhang, R. P. Deng, W. Hu, D. P. Liu, S. Y. Song and H. J. Zhang, CrystEngComm, 2011, 13, 4903–4908 RSC.
- Y. Huang, X. L. Huang, J. S. Lian, D. Xu, L. M. Wang and X. B. Zhang, J. Mater. Chem., 2012, 22, 2844–2847 RSC.
- X. H. Wang, X. W. Li, X. L. Sun, F. Li, Q. M. Liu, Q. Wang and D. Y. He, J. Mater. Chem., 2011, 21, 3571–3573 RSC.
- Y. Q. Zou and Y. Wang, Nanoscale, 2011, 3, 2615–2620 RSC.
- S. L. Xiong, C. Z. Yuan, X. G. Zhang and Y. T. Qian, CrystEngComm, 2011, 13, 626–632 RSC.
- S. J. Ding, T. Zhu, J. S. Chen, Z. Y. Wang, C. L. Yuan and X. W. Lou, J. Mater. Chem., 2011, 21, 6602–6606 RSC.
- B. Zhao, J. S. Song, P. Liu, W. W. Xu, T. Fang, Z. Jiao, H. J. Zhang and Y. Jiang, J. Mater. Chem., 2011, 21, 18792–18798 RSC.
- C. Y. Ge, Z. H. Hou, B. H. He, F. Y. Zeng, J. G. Cao, Y. M. Liu and Y. F. Kuang, J. Sol-Gel Sci. Technol., 2012, 63, 146–152 CrossRef CAS PubMed.
- X. F. Li, A. Dhanabalan, K. Bechtold and C. L. Wang, Electrochem. Commun., 2010, 12, 1222–1225 CrossRef CAS PubMed.
- X. F. Li, A. Dhanabalan and C. L. Wang, J. Power Sources, 2011, 196, 9625–9630 CrossRef CAS PubMed.
- K. Liang, X. Z. Tang and W. C. Hu, J. Mater. Chem., 2012, 22, 11062–11067 RSC.
- W. Ni, F. X. Liang, J. G. Liu, X. Z. Qu, C. L. Zhang, J. L. Li, Q. A. Wang and Z. Z. Yang, Chem. Commun., 2011, 47, 4727–4729 RSC.
- X. Xu, J. Liang, H. Zhou, D. M. Lv, F. X. Liang, Z. L. Yang, S. J. Ding and D. M. Yu, J. Mater. Chem. A, 2013, 1, 2995–2998 CAS.
- X. Xu, G. R. Yang, J. Liang, S. J. Ding, C. L. Tang, H. H. Yang, W. Yan, G. D. Yang and D. M. Yu, J. Mater. Chem. A, 2014, 2, 116–122 RSC.
- G. Q. Zhang, L. Yu, H. E. Hoster and X. W. Lou, Nanoscale, 2013, 5, 877–881 RSC.
- X. J. Zhang, W. H. Shi, J. X. Zhu, W. Y. Zhao, J. Ma, S. Mhaisalkar, T. L. Maria, Y. H. Yang, H. Zhang, H. H. Hng and Q. Y. Yan, Nano Res., 2010, 3, 643–652 CrossRef CAS PubMed.
- S. I. Kim, J. S. Lee, H. J. Ahn, H. K. Song and J. H. Jang, ACS Appl. Mater. Interfaces, 2013, 5, 1596–1603 CAS.
- Y. G. Wang and Y. Y. Xia, Electrochim. Acta, 2006, 51, 3223–3227 CrossRef CAS PubMed.
- S. Vijayakumar, S. Nagamuthu and G. Muralidharan, ACS Appl. Mater. Interfaces, 2013, 5, 2188–2196 CAS.
| This journal is © The Royal Society of Chemistry 2014 |