In situ engineering of urchin-like reduced graphene oxide–Mn2O3–Mn3O4 nanostructures for supercapacitors
Abstract
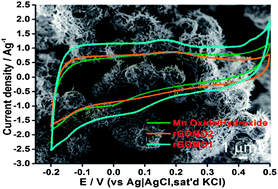
We report the use of a spray pyrolysis method to synthesize high surface area (BET surface area of 139 m2 g−1) self-organized, micron sized urchin-like composites made up of reduced graphene oxide and needle-shaped manganese oxide (rGO–Mn2O3–Mn3O4). Maximum capacitances of 425 Fg−1 at 5 mV s−1 from a three electrode set up and 133 Fg−1 at a current density of 0.2 Ag−1 were recorded using an asymmetric two electrode set up with graphene as the anode. The composite material also showed a capacitance retention of 83% over 1000 cycles. We attribute this remarkable performance to the high specific surface area due to the urchin-like hollow structures and synergy between the manganese oxide and reduced graphene oxide materials within the composite. Furthermore, this synthesis technique can be exploited further in the bulk synthesis of cost effective graphene–metal oxide hybrid materials for energy storage applications.

 Please wait while we load your content...
Please wait while we load your content...