Coumarin-derived Fe3+-selective fluorescent turn-off chemosensors: synthesis, properties, and applications in living cells†
Da
En
a,
Yuan
Guo
*ab,
Bo-Ting
Chen
c,
Biao
Dong
c and
Meng-Jiao
Peng
a
aKey Laboratory of Synthetic and Natural Functional Molecule Chemistry of the Ministry of Education, College of Chemistry and Materials Science, Northwest University, 229 Taibai Road (North), Xi'an 710069, P. R. China. E-mail: guoyuan@nwu.edu.cn; Fax: +86 29 8830 2122; Tel: +86 29 8830 2122
bInstitut de Chimie Organique et Analytique, Université d'Orléans, 45067 Orléans cedex 2, France
cState Key Laboratory on Integrated Optoelectronics, College of Electronic Science and Engineering, Jilin University, Changchun 130012, P. R. China
First published on 7th November 2013
Abstract
A novel coumarin-based fluorogenic probe bearing the tris unit (DAT-1) with high selectivity and suitable affinity toward Fe3+ over other cations tested was developed in biological systems. The sensing mechanism was studied by quantum calculations. The probe can be applied to the monitoring of Fe3+ in aqueous solution with a pH span of 3–8. In addition, biological imaging, membrane permeability and nontoxicity demonstrate that DAT-1 could act as a turn-off fluorescent chemosensor for Fe3+ in living cells.
Introduction
The design and synthesis of high selectivity and sensitivity chemosensors to detect metal ions in the ecological environment and biology have attracted a great deal of attention.1 A chemosensor shows a significant change in electrical, electronic, magnetic, or optical signal when it binds to a specific guest counterpart.2 Current chemosensors have many advantages, including high sensitivity, low cost, easy detection, and suitability as a diagnostic tool for biological purposes.3 As an essential trace element in biological systems, Fe3+ plays an important role in living organisms and metabolism. Many enzymatic reactions involving iron are related to oxygen metabolism, electron transfer and the mitochondrial respiratory chain.4 Although iron is indispensable for the proper functioning of all living cells, it is disadvantageous when present in excess. Excessive Fe3+ in the human body has been found to be related to an increased incidence of certain cancers and the dysfunction of certain organs.5 Moreover, hereditary hemochromatosis is characterized by excess iron that causes tissue damage and fibrosis with irreversible damage to various organs.4a,6 Furthermore, iron homeostasis is an important factor involved in neuroinflammation and the progression of Alzheimer's disease.4a,7 At the same time, iron deficiency can be as harmful as iron overload, or even more harmful. It is well known that anemia is due to iron deficiency. Therefore, a convenient and rapid method for detecting the concentration of Fe3+ in biological samples is of great interest in biological and environmental concerns. Recently, many fluorescent chemosensors for Fe(III)-selective detection were reported and have been used with some success in biological applications.8 Nevertheless, a few of them have defects in actual practice such as cross-sensitivity toward other metal cations, long response times, poor water solubility, a narrow pH detection span, a low fluorescence quantum yield in aqueous media, and ligand cytotoxicity.9Of late, coumarin-based chemosensors have been extensively used as fluorescent labeling reagents for their large molar extinction coefficient, relatively long excitation and emission wavelengths and high fluorescence quantum yield.10 Here, we synthesized a novel coumarin-derived Fe(III)-selective chemo-sensor N-[2-hydroxy-1,1-bis(hydroxymethyl)ethyl]-7-hydroxycoumarin-3-carboxamide (DAT-1) which is highly selective and sensitive toward Fe3+ over other common metal ions such as Na+, K+, Ca2+, Fe2+, Mg2+, Al3+, Co2+, Ni2+, Ag+, Cu2+, Zn2+, Cr3+, Cd2+, Mn2+, Hg2+ and Pb2+ in buffered aqueous solutions, and the sensing mechanism was studied by quantum calculations.
Experiment
Ethyl-7-hydroxy-3-carboxylate 2 (ref. 10) was synthesized according to the procedures reported in the literature. 2-Amino-2-(hydroxymethyl)-3-propanediol (tris) was purchased. DAT-1 was prepared by the standard method with a minor modification.11 Compound 2 (234 mg, 1 mmol) was treated with tris (121 mg, 1 mmol) in anhydrous ethanol (5 mL). The reaction was then refluxed for 8 h. The mixture was concentrated under vacuum and the crude product was purified by column chromatography (CH2Cl2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CH3OH = 10
CH3OH = 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to give DAT-1 as a yellow solid (185 mg, yield: 60 %), mp. 239–241 °C. 1HNMR (400 MHz, DMSO-d6): δ = 11.09 (s, 1H), 8.96 (s, 1H), 8.81 (s, 1H), 7.81 (d, J = 8.5 Hz, 1H), 6.89 (dd, J = 8.5, 1.7 Hz, 1H), 6.82 (d, J = 1.6 Hz, 1H), 4.86 (s, 3H), 3.65 (s, 6H). 13CNMR (400 MHz, DMSO-d6): δ 169.08, 166.84, 166.39, 161.52, 153.23, 137.19, 119.62, 118.82, 116.19, 106.96, 67.44, 65.30, 45.27, 45.06, 44.86, 44.65, 44.44, 44.23, 44.02. HRMS (ESI): m/z, calcd for (M − H)− 308.0770; found 308.0798 (Scheme 1).
1) to give DAT-1 as a yellow solid (185 mg, yield: 60 %), mp. 239–241 °C. 1HNMR (400 MHz, DMSO-d6): δ = 11.09 (s, 1H), 8.96 (s, 1H), 8.81 (s, 1H), 7.81 (d, J = 8.5 Hz, 1H), 6.89 (dd, J = 8.5, 1.7 Hz, 1H), 6.82 (d, J = 1.6 Hz, 1H), 4.86 (s, 3H), 3.65 (s, 6H). 13CNMR (400 MHz, DMSO-d6): δ 169.08, 166.84, 166.39, 161.52, 153.23, 137.19, 119.62, 118.82, 116.19, 106.96, 67.44, 65.30, 45.27, 45.06, 44.86, 44.65, 44.44, 44.23, 44.02. HRMS (ESI): m/z, calcd for (M − H)− 308.0770; found 308.0798 (Scheme 1).
Results and discussion
The DAT-1 chemosensor contain a coumarin group used as the fluorescent signal unit and a 2-amino-2-(hydroxymethyl)-1,3-propanediol (tris) acting as a possible recognition unit. Since coumarin derivatives exhibit several advantages such as large Stokes shifts, good photostability and high fluorescence quantum yields (the quantum yield of the system was calculated to be 0.75), they could be used as efficient fluorophores. And the tris which includes three hydroxyl groups and an amide group is expected to serve as a potential binding group.11 Furthermore, the tris group can improve the water solubility of chemosensors due to its hydrophilic properties.The structure of DAT-1 was confirmed by 1H NMR, 13C NMR, FT-IR, ESI-MS and X-ray analysis (Fig. S7–S10†). The single crystal of DAT-1 was obtained under vapor diffusion of CH3OH into a solution of water and characterized using X-ray crystallography (Fig. 1).
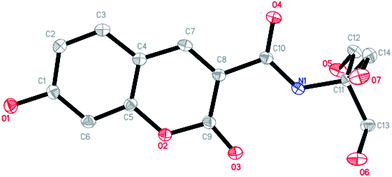 | ||
| Fig. 1 View of the structure of DAT-1 with displacement atomic ellipsoids drawn at the 30% probability level. All hydrogen atoms are omitted for clarity. | ||
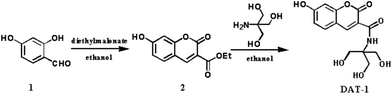
Fig. 2a shows the change in the fluorescence spectra of DAT-1 upon the addition of Fe3+ in the Na2HPO4–citric acid buffer solution at pH 4.8. Upon the addition of an increasing amount of Fe3+, the emission intensity of DAT-1 at 448 nm gradually decreased. The fluorescence emission intensity were sensitively and proportionately decreased with an increasing concentration of Fe3+ as noted from the relationship between the fluorescence emission intensity at 350 nm and Fe3+ concentration (Fig. 2b). Therefore, the DAT-1 could be used for the development of an efficient method for Fe3+ ion detection. The linear relation of the obtained experimental data for Fe3+ detection could be best described as the Stern–Volmer equation. The Stern–Volmer relationship with an R2 of 0.996 is given in the following equation (Fig. 2b, inset):
| I0/I = 1 + KSV[Q] |
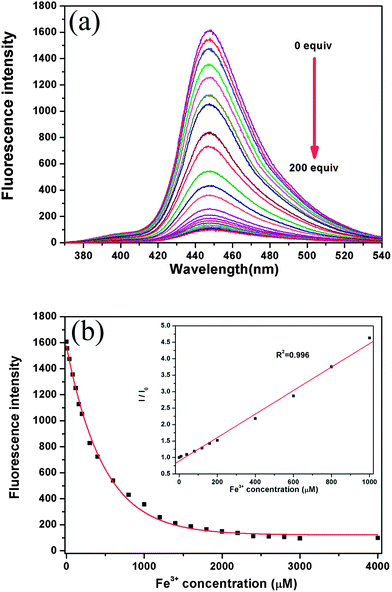
There was a remarkable selectivity of DAT-1 for Fe3+ over various other metal ions in the competition experiments, which turned out to be applicable in environmental technology (Fig. 3). The fluorescence intensity of the chemosensor DAT-1 was substantially diminished by the addition of Fe3+, whereas other cations such as K+, Ca2+, Fe2+, Mg2+, Al3+, Co2+, Ni2+, Ag+, Cu2+, Zn2+, Cr3+, Cd2+, Mn2+, Hg2+ and Pb2+ did not cause any significant changes in the fluorescence emission intensity, even at a concentration of 100 equiv. of guest counterparts. Even with highly concentrated Na+, K+, Ca+, and Mg2+ (∼5.0 mM) under physiological conditions,12 there were no fluorescence changes in DAT-1. The large decrease in fluorescence intensity could be applied to the detection of iron cations by the naked eye (Fig. 4). When the chemosensor (20 μM) was excited at 350 nm in the presence of various cations (100 equiv.) in Na2HPO4–citric acid buffer (pH 4.8), hardly any fluorescence response of Fe3+ was selectively observed (Fig. 4).
 | ||
| Fig. 4 Fluorescent (bottom) and color (top) responses of DAT-1 (20 μM) in Na2HPO4–citric acid buffer solutions (0.2 M) upon the addition of (100 equiv.) metal ions in water. | ||
It is well-known that heavy metal ions such as Fe3+, Cu2+, Cd2+, Hg2+ and Pb2+ tend to quench the luminescence through electron- and/or energy-transfer processes.13 Because of the specific structure, the recognition unit of our probe could complex with iron ions selectively. Thus the photoinduced electron transfer (PET) effect on N atom causes fluorescence quenching of coumarin. The fact that our DAT-1 chemosensor can not complex with other ions besides iron ions is attributed to the specific recognition of the recognition unit to iron ions.13e Therefore, our probe cannot be quenched by other ions without PET effect. To prove the mechanism of fluorescence quenching, we carried out theoretical calculations (Fig. 5; details in Fig. S3†). From the single crystal structure of DAT-1, there are many coordination sites, but it is difficult to determine the structure of coordination between DAT-1 and Fe3+. We therefore designed 22 coordinations, considering all possible options except water as ligand. We found, however, that complex-23, with water as ligand, was the optimum structure compared with other complexes. This result agrees with the experimental data reported by other authors.11 Thus we can assume that complex-23 was the best structure in this system. The electron distributions of HOMO, LUMO, and LUMO+4 of complex-23 were calculated by B3LYP/6-31G(d, p) and are shown in Fig. 5. The electronic distribution on LUMO+4 for complex-23 was extended to Fe, indicating the electron transfer from HOMO to LUMO+4. Thus the electrons transfer from DAT-1 to Fe3+, making the fluorescence quenching behavior possible. Indeed, it has been demonstrated in the literature that fluorescence quenching of the ligand may occur by the excitation energy transfer from the ligand to the metal d orbital and/or ligand to metal charge transfer (LMCT).9,14
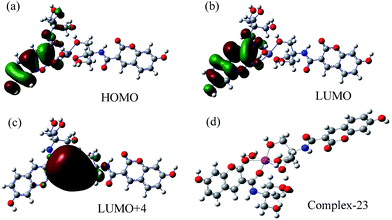 | ||
| Fig. 5 The calculated frontier molecular orbitals of complex-23 (a–c) and the best structure of complex-23 (d). | ||
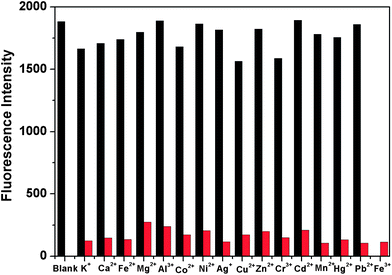
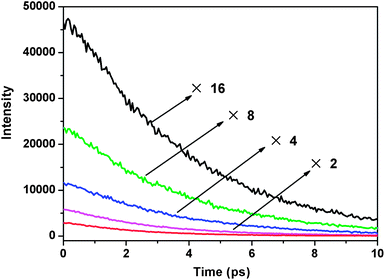
To elaborate upon the mechanism of fluorescence quenching which we speculate takes place by the energy and/or charge transfer model, fluorescence lifetime testing was conducted. The fluorescence decay behavior in the presence of Fe3+ is shown in Fig. 6, and the exponential fit results are summarized in Table 1. In the absence of Fe3+, the fluorescence decays single exponentially by a 3.68 ns time constant, which is the lifetime of the S1 state of free DAT-1. The time constant (τ) of the lifetime decay component drops off when Fe3+ is added.
| Fe3+ (equiv.) | τ (ns) | Fe3+ (equiv.) | τ (ns) |
|---|---|---|---|
| 0 | 3.68 | 25 | 2.76 |
| 1 | 3.60 | 50 | 2.30 |
| 5 | 3.42 |
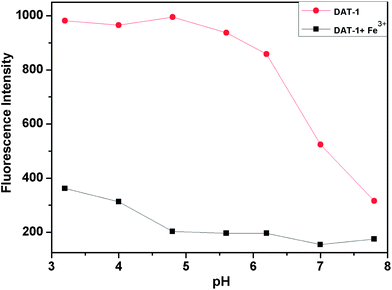
For biological applications of the chemosensor, the sensing should operate in a wide range of pH. Fig. 7 shows that in aqueous solution the suitable pH range for Fe3+ determination is pH 3–8 where the “on–off” fluorescence can be operated by the iron ion binding. Consequently, our present Fe3+-selective receptor would be an ideal chemosensor for monitoring Fe3+.
The ability of biosensing molecules to selectively monitor guest species in living cells is of great importance for biological applications. First, we selected breast carcinoma cell lines (MCF-7) to investigate the toxicity of DAT-1. The toxicity data were obtained by performing 3-(4,5-dimethylthiazol-2-yl)-2,5 diphenyltetrazolium bromide (MTT) assays as shown in Fig. 8. The cells were cultured in RPMI 1640 (Gibco) and supplemented with 10% fetal bovine serum (FBS; Gibco) at 37 °C in a humidified atmosphere of 5% CO2. For all experiments, cells were harvested from subconfluent cultures by the use of trypsin and were resuspended in fresh complete medium before plating. In vitro cytotoxicity was assessed by using MTT reduction assays. In a typical procedure, about 2000 cells were plated in 96-well plates for 24 h to allow the cells to attach, and then incubated with DAT-1 (20 μM) in 5% CO2 at 37 °C. At the end of the incubation time, MTT solution (10 μL, diluted in culture medium to a final concentration of 1 mg mL−1) was added, and then the mixture was incubated for another 4 h. Finally, the incubation solution was removed and dimethyl sulfoxide (DMSO, 150 μL) was added to each well. The absorbance of MTT was determined (λ = 490, 630 nm) by using a microplate reader (BioTek, ELX808). The cell viability obtained was expressed as a percentage relative to the control. The viability of untreated cells was assumed to be 100%. When the concentration of chemosensor DAT-1 was 5 μM, more than 99% of the MCF-7 cells were alive. Even when the concentration of DAT-1 increased to 100 μM, there were still about 87% of the MCF-7 cells alive. From the results of cytotoxicity experiments, the chemosensor DAT-1 shows low toxicity even at a high concentration of 100 μM particles and an incubation time of 24 h, which means that DAT-1 possesses high biocompatibility.
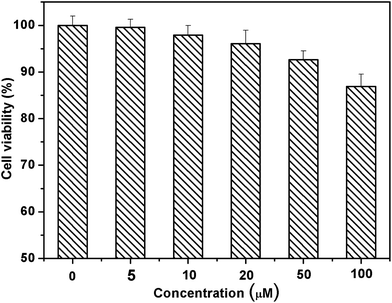 | ||
| Fig. 8 Percentage of MCF-7 cell viability remaining after cell treatment with DAT-1 (untreated cells were considered to have 100% survival). Cell viability was assayed by the MTT method (values: mean ± standard deviation).15 | ||
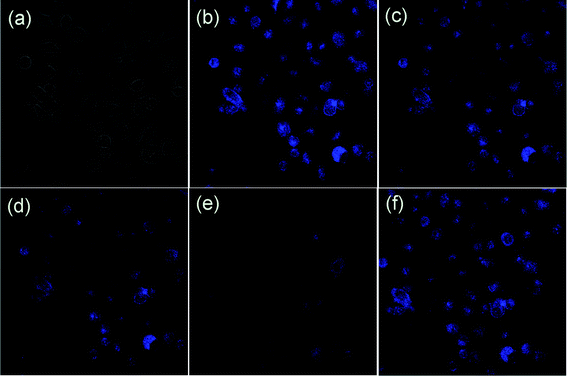
To determine the cell permeability of DAT-1, MCF-7 cells were incubated with DAT-1 (20 μM) for 25–30 min at 37 °C, and washed with PBS to remove the remaining compound DAT-1. The results are shown in Fig. 9b. One can clearly observe significant confocal imaging changes of the medium upon addition of FeCl3 for 20 min (25, 50, and 100 equiv., respectively) at 37 °C. MCF-7 cells incubated with DAT-1 initially display a strong fluorescence image, but the fluorescence image immediately becomes faint in the presence of Fe3+ (Fig. 9c–e). Thus, the chemosensor DAT-1 can be a suitable fluorescence chemosensing probe for Fe3+ detection in biological systems.
Conclusion
In summary, this study has reported the synthesis, properties, and cellular applications of a novel chemosensor DAT-1 for Fe3+ sensing. This chemosensor exhibits high sensitivity and selectivity toward Fe3+ in aqueous solution. We simulated the mechanism of fluorescence quenching by quantum calculations. In terms of good optical properties, fine water solubility, excellent membrane permeability and non-toxicity, DAT-1 could be one of the most important chemosensors for the detection of Fe(III) in living cells.Acknowledgements
We are very grateful for the support of this work from the National Natural Science Foundation of China (no. 21072158 and 20802056), the Special Foundation of the Education Committee of Shaanxi Province (no. 12JK0580), the French Chinese Foundation for Science and Applications (FFCSA) and the China Scholars Council (CSC).Notes and references
- (a) D. H. Vance and A. W. Czarnik, J. Am. Chem. Soc., 1994, 116, 9397–9398 CrossRef CAS; (b) Z. C. Xu, J. Yoon and D. R. Spring, Chem. Soc. Rev., 2010, 39, 1996–2006 RSC; (c) J. S. Wu, W. M. Liu, J. C. Ge, H. Y. Zhang and P. F. Wang, Chem. Soc. Rev., 2011, 40, 3483–3495 RSC; (d) M. Baglan and S. Atilgan, Chem. Commun., 2013, 49, 5325–5327 RSC; (e) M. J. Peng, Y. Guo, X. F. Yang, L. Y. Wang and J. An, Dyes Pigm., 2013, 98, 327–332 CrossRef CAS PubMed.
- (a) T. W. Bell and N. M. Hext, Chem. Soc. Rev., 2004, 33, 589–598 CAS; (b) W. Wang, X. L. Wang, Q. B. Yang, X. L. Fei, M. D. Sun and Y. Song, Chem. Commun., 2013, 49, 4833–4835 RSC; (c) X. G. Li, Y. Z. Liao, M. R. Huang, V. Strong and R. B. Kaner, Chem. Sci., 2013, 4, 1970–1978 RSC; (d) Z. Yang, M. Y. She, B. Yin, J. H. Cui, Y. Z. Zhang, W. Sun, J. L. Li and Z. Shi, J. Org. Chem., 2012, 77, 1143–1147 CrossRef CAS PubMed.
- (a) C. Y. Lai, B. G. Trewyn, D. M. Jeftinija, K. Jeftinija, S. Xu, S. Jeftinija and V. S. Y. Lin, J. Am. Chem. Soc., 2003, 125, 4451–4459 CrossRef CAS PubMed; (b) P. G. Sutariya, A. Pandya, A. Lodha and S. K. Menon, Analyst, 2013, 138, 2531–2535 RSC; (c) G. Sivaraman, T. Anand and D. Chellappa, Analyst, 2012, 137, 5881–5884 RSC; (d) X. J. Wu, Z. Li, L. Yang, J. H. Han and S. F. Han, Chem. Sci., 2013, 4, 460–467 RSC.
- (a) S. K. Sahoo, D. Sharma, R. K. Bera, G. Crisponi and J. F. Callan, Chem. Soc. Rev., 2012, 41, 7195–7227 RSC; (b) S. P. McCormick, M. Chakrabarti, A. L. Cockrell, J. Park, L. S. Lindahl and P. A. Lindahl, Metallomics, 2013, 5, 232–241 RSC.
- (a) B. J. Halliwell, J. Neurochem., 1992, 59, 1609–1623 CrossRef CAS; (b) E. D. Weinberg, Eur. J. Cancer Prev., 1996, 5, 19–36 CAS; (c) D. Galaris, V. Skiada and A. Barbouti, Cancer Lett., 2008, 266, 21–29 CrossRef CAS PubMed.
- E. Beutler, Blood Cells, Mol., Dis., 2007, 39, 140–147 CAS.
- W. Y. Ong and A. A. Farooqui, J. Alzheimer's Dis., 2005, 8, 183–200 CAS.
- (a) L. Huang, F. P. Hou, J. Cheng, P. X. Xi, F. J. Chen, D. Bai and Z. Z. Zeng, Org. Biomol. Chem., 2012, 10, 9634–9638 RSC; (b) S. K. Sahoo, D. Sharma, R. K. Bera, G. Crisponi and J. F. Callan, Chem. Soc. Rev., 2012, 41, 7195–7227 RSC; (c) B. K. Kanungo, M. Baral, R. K. Bera and S. K. Sahoo, Monatsh. Chem., 2010, 141, 157–168 CrossRef CAS.
- H. S. Jung, P. S. Kwon, J. W. Lee, J. I. Kim, C. S. Hong, J. W. Kim, S. H. Yan, J. Y. Lee, J. H. Lee, T. H. Joo and J. S. Kim, J. Am. Chem. Soc., 2009, 131, 2008–2012 CrossRef CAS PubMed.
- K. Y. Chen, Y. Guo, Z. H. Lu, B. Q. Yang and Z. Shi, Chin. J. Chem., 2010, 28, 55–60 CrossRef CAS.
- J. N. Yao, W. Dou, W. W. Qin and W. S. Liu, Inorg. Chem. Commun., 2009, 12, 116–118 CrossRef CAS PubMed.
- T. D. Rae, P. J. Schmidt, R. A. Pufahl, V. C. Culotta and T. V. O'Halloran, Science, 1999, 284, 805–808 CrossRef CAS.
- (a) J. L. Bricks, A. kovalchuk, C. Trieflinger, M. Nofz, M. Buschel, A. I. Tolmachev, J. Daub and K. Rurack, J. Am. Chem. Soc., 2005, 127, 13522–13529 CrossRef CAS PubMed; (b) K. Kavallieratos, J. M. Rosenberg, W. Z. Chen and T. Ren, J. Am. Chem. Soc., 2005, 127, 6514–6515 CrossRef CAS PubMed; (c) Y. Zheng, J. Orbulescu, X. Ji, F. M. Andreopoulos, S. M. Pham and R. M. Leblanc, J. Am. Chem. Soc., 2003, 125, 2680–2688 CrossRef CAS PubMed; (d) D. T. Quang, H. S. Jung, J. H. Yoon, S. Y. Lee and J. S. Kim, Bull. Korean Chem. Soc., 2007, 28, 682 CrossRef CAS; (e) T. E. Glass, J. Am. Chem. Soc., 2000, 122, 4522–4523 CrossRef CAS.
- L. S. Villata, E. Wolcan, M. R. Feliz and A. L. Capparelli, J. Phys. Chem. A, 1999, 103, 5661–5666 CrossRef CAS.
- (a) Y. Cetin and L. B. Bullerman, J. Agric. Food Chem., 2005, 53, 6558–6563 CrossRef CAS PubMed; (b) I. Roy, T. Y. Ohulchanskyy, H. E. Pudavar, E. J. Bergey, A. R. Oseroff, J. Morgan, T. J. Dougherty and P. N. Prasad, J. Am. Chem. Soc., 2003, 125, 7860–7865 CrossRef CAS PubMed; (c) S. Andreescu, O. A. Sadik and D. W. McGee, Chem. Res. Toxicol., 2005, 18, 466–474 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 939460. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c3ra44843f |
| This journal is © The Royal Society of Chemistry 2014 |