Facile one-pot synthesis of Au nanoparticles decorated porous α-Fe2O3 nanorods for in situ detection of VOCs†
Shurong
Wang
*,
Hongxin
Zhang
,
Yanshuang
Wang
,
Liwei
Wang
and
Zhe
Gong
Tianjin Key Lab of Metal and Molecule-based Material Chemistry, Department of Chemistry, Nankai University, Tianjin, 300071, China. E-mail: shrwang@nankai.edu.cn; Fax: +86-22-23502458; Tel: +86-22-23505896
First published on 8th November 2013
Abstract
Porous Au/α-Fe2O3 NRs are one-pot synthesized, and used as gas sensors for in situ VOCs detection. The sensor shows four times higher response to C6H6 than a pure α-Fe2O3 sensor and short response/recovery time (<30 s), which may be useful to establish an effective C6H6 real-time monitoring system in environmental protection and oil reservoirs' prediction.
Volatile organic compounds (VOCs), widely used in industrial, residential and commercial processes, are considered as an important class of environmental pollutants in that their existence in the atmosphere may cause severe safety or health problems due to their toxic or hazardous characteristics, attracting public and academic attention.1,2 To provide adequate health and environmental protection, it is essential to establish a fast, simple, reliable and effective VOCs real-time monitoring system.
The gas sensor, as an innovative detection mode, has been developed to detect various toxic or hazardous gases or vapors in the last few decades. α-Fe2O3, a typical n-type semiconductor metal oxide (SMO), has attracted great attention for its potential application as a promising gas sensing material in detecting ethanol, acetone, H2, and CH4.3–5 Recently, many attempts have been made to enhance the gas sensing performances of α-Fe2O3 based gas sensors. The deposition of noble metal nanoparticles (NPs), such as Au, Pd, and Pt, onto SMO nanostructures has been demonstrated to be effective to improve the physical and chemical performance of SMO.6–9 Among them, Au based hybrid nanomaterials have received special attention since Haruta et al. reported that Au NPs with small particle size supported on transition metal oxides, such as α-Fe2O3, TiO2, and Co3O4, were very active for low temperature CO oxidation.10 To date, it has been reported that the Au-containing catalysts exhibited a high catalytic activity in various important reactions including CO and H2 oxidation, NO reduction, CO2 hydrogenation, water–gas shift reaction, selective liquid phase hydrogenation of citral, and catalytic combustion of methanol and were used to study metal-support interaction and catalytic reaction mechanism.11–15 These studies have demonstrated that the small particle size and the interaction between Au and support are responsible for the high-catalytic activity. Au/α-Fe2O3 system has also been researched in several other applications including gas sensors,6–8 selectively induction of apoptosis in cancer cells,16 and electrochemical DNA biosensor for Escherichia coli detection.17 As for gas sensor, in the last years, there has been a growing interest in Au-decorated or doped sensing materials, because Au could significantly enhance the performances of gas sensors due to its exceptional catalytic activity.6–8
In addition, several recent reports have shown that the gas sensing performance of α-Fe2O3 based sensors strongly depend on the size, specific surface area, and morphology. 1D α-Fe2O3 nanostructures such as nanowires or nanorods, have been considered as ideal building blocks for constructing nanoscale sensors due to their high surface-to-volume ratio and the special physical and chemical properties originating from their size.18–20 Potentially, α-Fe2O3 NRs decorated with Au NPs can combine 1D nanostructure of support and the unique catalytic property of Au NPs, which will help to find novel properties and enlarge the applications of such functional hybrid nanomaterials.
Because the morphology of Au NPs and their distribution on the surface of α-Fe2O3 are critical to the Au catalytic effect,7,21–23 it is highly desired to uniformly modify the surfaces of NRs with small size Au NPs, which would provide a larger noble metal–semiconductor interface area and allow for more efficient electronic interaction between noble metal and semiconductor. However, the controlled synthesis of highly dispersed Au NPs on the surfaces of α-Fe2O3 still faces tremendous challenges. Inconveniently, traditional methods to prepare 1D nanomaterials and noble metal decorated or doped hybrids often require two steps or more and induce impurities in the final products when templates or capped agents are introduced into the reaction system. Therefore, a simple, facile and effective solution-based synthetic strategy should be adopted.
Herein, Au decorated porous α-Fe2O3 NRs (Au/α-Fe2O3 NRs) were synthesized by a simple one-pot low temperature hydrothermal strategy only using FeSO4·7H2O, CH3COONa and HAuCl4·4H2O as starting materials, without structure directing agent and Au capped agent used (Experimental section in ESI†).
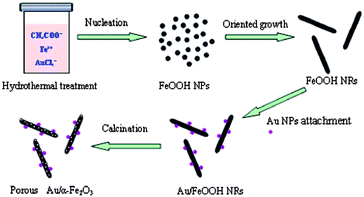
As shown in Scheme 1, formation of the porous Au/α-Fe2O3 NRs involves several steps: firstly, in the initial stage, OH− was produced by the hydrolysis of CH3COO− under the hydrothermal condition, as shown in the eqn (1),24 and the primary FeOOH nanocrystals were rapidly formed by the reaction of Fe2+ with O2 and OH− (eqn (2)).24 Then, due to the thermodynamical unstablility resulted from the high surface energy, these small primary FeOOH NPs tend to oriented aggregate and further grow to rod-like nanostructures. Meanwhile, under the hydrothermal condition, the chloroauric acid (AuCl4−) ions can be reduced by Fe2+ in the solution to produce Au NPs (eqn (3)), which are anchored onto the surface of FeOOH NRs. Finally, the porous Au/α-Fe2O3 NRs were obtained by calcination of Au/FeOOH precursors without deformation in structure. The decomposition of FeOOH and the release of gas in confined space during the thermal process lead to the formation of the porous structure.
| CH3COO− + H2O → CH3COOH + OH− | (1) |
| 4Fe2+ + 8OH− + O2 → 4FeOOH + 2H2O | (2) |
| AuCl4− + 3Fe2+ →Au + 3Fe3+ +4Cl− | (3) |
| 2FeOOH → α-Fe2O3 + H2O | (4) |
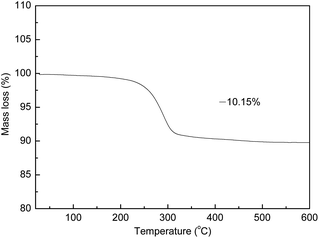
TGA was performed to study the decomposition process of the as-prepared Au doped FeOOH precursor during calcination, and the result is shown in Fig. 1. It can be clearly observed from Fig. 1 that there is an abrupt mass loss step from 200–300 °C, which is ascribed to the decomposition of FeOOH precursor, and no obvious mass loss occurs above 300 °C. The total mass loss is about 10.15%, which is almost equal to the theoretical mass loss value (10.11%) of FeOOH decomposed into α-Fe2O3, indicating the pure α-Fe2O3 phase is formed after calcination. This result is well consistent with the below XRD analysis results.
Evidence of the phase composition evolution is given by XRD. Fig. 2 illustrates the XRD patterns of the samples before and after calcination. The main diffraction peaks of the precursor before calcination (curve a) can be indexed as the goethite FeOOH phase, which is consistent with the JCPDS file no. 29-0713. As for the product after calcination at 300 °C, all of the diffraction deflection peaks (curve b) are in well agreement with the standard data of α-Fe2O3 (JCPDS file no. 33-0664). No characteristic peaks are observed for impurities such as FeO, γ-Fe2O3 and Fe3O4, indicating that the phase of goethite FeOOH is completely transformed into the phase of α-Fe2O3 after calcination at 300 °C. However, in the both patterns, no characteristic peaks are related to the Au phase, such as at 2θ = 38.2°, 44.5° and 64.6°, ascribed to the (111), (200) and (220) planes of face-centered cubic (fcc) Au (JCPDS file no. 01-1174), respectively, can be detected. The absence of the Au characteristic peaks may be due to the high dispersion of the Au nanoparticles with too small particle sizes on the surface of the support to be identified by the conventional XRD method. This is also confirmed by the below-mentioned XPS, TEM and EDS measurements.
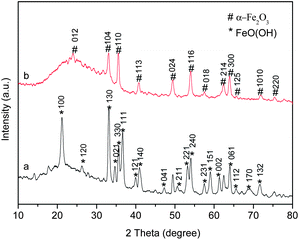 | ||
| Fig. 2 XRD patterns of the precursor before calcination (curve a) and the product after calcination (curve b). | ||
The surface chemical compositions and the valence states of the product were revealed by XPS (Fig. 3). The wide XPS spectrum shown in Fig. 3A confirms the existence of Au, Fe and O elements. To identify all the states of Au, Fe and O elements, the high resolution spectra of Au 4f, Fe 2p and O 1s are illustrated in Fig. 3B–D, respectively. Fig. 3B presents the XPS spectrum of Au 4f doublet peaks, and the binding energy of Au 4f5/2 and Au 4f7/2 is observed at approximately 88.1 and 84.2 eV, respectively, with the splitting data between the Au 4f5/2 and Au 4f7/2 core levels are 3.9 eV, indicating clearly the presence of gold in the metallic state, which is well consistent with the reported literature.10,25,26 The Fe 2p core level spectrum is displayed in Fig. 3C, showing the main peaks of Fe 2p3/2 and Fe 2p1/2 at the binding energy of 710.9 and 724.5 eV, respectively, together with a weak satellite peak between 715 and 720 eV, which corresponds to the Fe3+ surface species in α-Fe2O3, indicating that iron is at the +3 state in the product.26–28Fig. 3D shows the XPS spectrum of the O 1s core-level, which is deconvoluted into three peaks centered at 530.0, 531.9 and 532.9 eV, respectively, indicating the existence of three different oxygen species. The low binding energy peak observed at 530.0 eV is well attributed to the O2− forming oxide with iron elements, and the high binding energy peak centered at 531.9 and 532.9 eV is assigned to O− of surface-adsorbed oxygen and O22− of surface-chemisorbed oxygen, respectively.29,30 In addition, according to the XPS analysis, the surface content of Au in the product is 0.77 wt%, which is higher than the theoretical value of 0.5 wt%, suggesting that the Au NPs are mainly well dispersed on the surface of the α-Fe2O3 NRs.
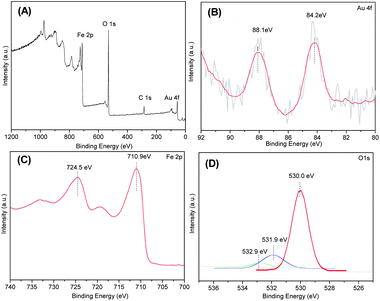 | ||
| Fig. 3 XPS spectra of AuNPs decorated α-Fe2O3 NRs: (A) wide spectrum and high resolution spectrum of (B) Au 4f, (C) Fe 2p and (D) O 1s. | ||
TEM images were obtained in order to investigate the structure of Au NPs decorated α-Fe2O3 NRs, to further confirm the successful decoration of Au NPs on the α-Fe2O3 NRs and to measure the particle size of Au NPs. The results are presented in Fig. 4. Low-magnification overview image in Fig. 4A shows clearly that the product has a rodlike morphology with a diameter of about 9–12 nm and a length of about 66–90 nm. Further examination of high-magnification TEM image in Fig. 4B reveals that a large amount of cavities can be clearly seen on the surface of α-Fe2O3 NRs, indicating the porous feature of the materials. The formation of the pores may be due to the removal of H2O in the calcination process of the goethite FeOOH precursor transformed into α-Fe2O3. These pores of the α-Fe2O3 NRs are expected to increase the accessible surface area and provide more active sites for the surface reactions. In addition, the loose and porous structure can also facilitate gas diffusion and mass transport in the process of surface reactions, thus improving the performances or properties of the materials based on the prepared α-Fe2O3 NRs.31–34 It is also clearly from Fig. 4B the Au NPs (indicated by green circles) with spherical shape have been formed and well decorated on the α-Fe2O3 NRs, and the Au NPs present a narrow size distribution between 3.7 and 9.0 nm, with an average diameter of ca. 6.6 nm (Fig. S2†). Importantly, all of the Au NPs are located on the surface of α-Fe2O3 NRs, suggesting that this method is favorable for preparing a highly dispersed Au NPs supported materials. The energy-dispersive X-ray spectrometry (EDS) (Fig. S3†) resulted from Fig. 4B displays the existence of Au, Fe and O elements, further confirming the successful assembly of Au NPs on the α-Fe2O3 NRs.
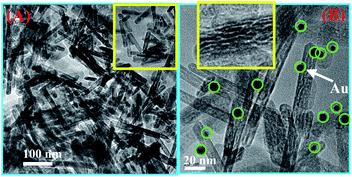 | ||
| Fig. 4 Low-magnification (A) and high-magnification (B) TEM images of the Au NPs decorated α-Fe2O3 NRs. | ||
To investigate the gas sensing properties of the sensor based on the AuNPs decorated α-Fe2O3 NRs, the gas sensing tests of several VOCs, including n-butanol, ethanol, methanol and benzene, were conducted. Fig. 5 depicts the histogram of the response of the sensors based AuNPs decorated α-Fe2O3 NRs and pure α-Fe2O3 NRs to 100 ppm of various gases. It can be seen from Fig. 5 that the sensor shows high response amplitudes to n-butanol, ethanol, methanol and benzene, with the response of 9.5, 8.7, 5.6 and 4.7, respectively, and a fast response-recovery characteristic for these VOCs, indicating its potential application in detecting VOCs. In can also be clearly seen that the sensor based on Au NPs decorated α-Fe2O3 NRs shows higher sensitivity to butanol, ethanol, methanol and benzene than the pure α-Fe2O3 NRs. Especially, the Au/α-Fe2O3 sensor shows almost four times higher response to benzene than pure α-Fe2O3 sensor. Except environmental contamination, it has been demonstrated that the presence of benzene in the soil could be an indicator of oil reservoirs.35,36 To predict the presence of oil reservoirs, the portable gas chromatograph devices have been used to detect benzene in the near surface layers of the earth. The gas chromatograph method is sensitive enough, but the long sample analysis times (above minutes) limit the area surveyed per day, so the shorted sampling time is welcome to survey much larger areas with more accuracy and in a time for oil reservoir and environmental contamination.35 In the present research, the response and recovery times are lower than 30 s. The fast response/recovery characteristic is essential for real-time monitoring of benzene and preventing possible disasters due to its toxicity.
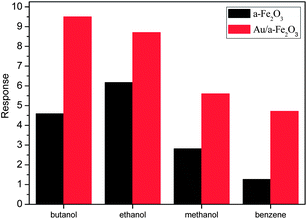 | ||
| Fig. 5 Response of the sensors based AuNPs decorated α-Fe2O3 NRs and pure α-Fe2O3 NRs to 100 ppm of various VOCs. | ||
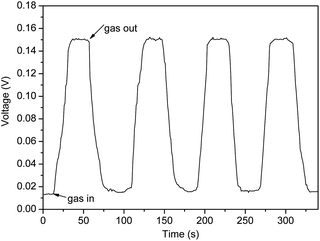
To evaluate the reproducibility for the VOC-sensing system, ethanol vapor was used as probe gas. A successive dynamic response–recovery sensing behavior of four cycles of the gas sensor based on AuNPs decorated α-Fe2O3 NRs to 100 ppm ethanol has been tested. As shown in Fig. 6, it can be clearly seen that, in the four cycles, the sensor exhibits only a neglectable change in response amplitude, indicating the good reproducibility of the AuNPs decorated α-Fe2O3 NR sensor.
The enhanced sensing performance of Au NPs decorated α-Fe2O3 NRs can be attributed to the following several reasons. Firstly, as an n-type semiconductor metal oxide (SMO) gas sensor material, α-Fe2O3 belongs to the surface-controlled type. It is widely accepted that oxygen could trap electrons from the conduction band of α-Fe2O3 to form adsorded oxygen species Oδ− (O2−, O− and O2−) in air. When the sensor is exposed to reductive gases, for instance, ethanol, the reductive gases would react with the adsorbed oxygen species, which results in the release of free electrons to the α-Fe2O3 sensor, thus leading to a decrease in the resistances. The prepared α-Fe2O3 materials in this work present unique one dimensional and porous microstructure, compared with the traditional α-Fe2O3 materials, which can bring about larger effective accessible surface area. This is anticipated to improve the formation of adsorded oxygen species, which can result in enhanced sensing performance. Secondly, after α-Fe2O3 NRs are decorated by Au NPs, the surface of the NRs are modulated by the chemical and electrical effect of Au NPs, which can make significant contributions to the enhanced gas sensing performance. Au NPs may serve as specific adsorption sites to dissolve oxygen molecules and adsorbed gas molecules.37,38 Compared with pure α-Fe2O3 NRs, there are more adsorbed oxygen species on the surface of the AuNPs decorated α-Fe2O3 NRs by capturing more free electrons from the conduction band of α-Fe2O3, which results in wider electron depletion.7,8,39–41 Thus the sensing reaction between adsorbed oxygen species and the tested gases would lead to a larger resistance change and a higher sensor response. Thirdly, the catalytic activity of Au NPs also contributes to the enhanced gas sensing performance, for the reaction between surface-adsorbed oxygen ions species and the reductive gases can be accelerated by the catalytic effect of Au NPs.
In summary, Au NPs decorated porous α-Fe2O3 NRs are easily synthesized by a one-pot low temperature hydrothermal method, without structure directing agent and Au capped agent used. Au/α-Fe2O3 NRs were used as gas sensing materials for in situ detection of several VOCs including n-butanol, ethanol, methanol and benzene. The sensor shows high response amplitudes and a fast response-recovery characteristic for these VOCs, indicating its potential application in detecting VOCs. Meanwhile, the sensor based on Au/α-Fe2O3 NRs shows higher response to these VOCs than the pure α-Fe2O3 NRs. Especially, the Au/α-Fe2O3 sensor shows almost four times higher response to benzene than a pure α-Fe2O3 sensor, which may be useful for establishing a fast, simple, reliable and effective benzene real-time monitoring system in environmental protection and predicting the presence of oil reservoirs.
Acknowledgements
The authors gratefully acknowledge the financial support from National Natural Science Foundation of China (no. 21171099 and 21301098) and 111 Project (B12015).Notes and references
- R. Atkinson and J. Arey, Chem. Rev., 2003, 103, 4605 CrossRef CAS PubMed.
- Z. Meng, D. Dabdub and J. H. Seinfeld, Science, 1997, 277, 116 CrossRef CAS.
- J. Chen, L. N. Xu, W. Y. Li and X. L. Gou, Adv. Mater., 2005, 17, 582 CrossRef CAS.
- S. R. Wang, L. W. Wang, T. L. Yang, X. H. Liu, J. Zhang, B. L. Zhu, S. M. Zhang, W. P. Huang and S. H. Wu, J. Solid State Chem., 2010, 183, 2869 CrossRef CAS PubMed.
- X. H. Liu, J. Zhang, S. H. Wu, D. J. Yang, P. R. Liu, H. M. Zhang, S. R. Wang, X. D. Yao, G. S. Zhu and H. J. Zhao, RSC Adv., 2012, 2, 6178 RSC.
- P. Gunawan, L. Mei, J. Teo, J. Ma, J. Highfield, Q. Li and Z. Zhong, Langmuir, 2012, 28, 14090 CrossRef CAS PubMed.
- X. H. Liu, J. Zhang, X. Z. Guo, S. H. Wu and S. Wang, Nanotechnology, 2010, 21, 095501 CrossRef PubMed.
- J. Zhang, X. H. Liu, X. Z. Guo, S. H. Wu and S. R. Wang, Chem.–Eur. J., 2010, 16, 8108 CrossRef CAS PubMed.
- Y. Wang, F. H. Kong, B. L. Zhu, S. R. Wang, S. H. Wu and W. P. Huang, Mater. Sci. Eng., B, 2007, 140, 98 CrossRef CAS PubMed.
- M. Haruta, N. Yamada, T. Kobayashi and S. Iijima, J. Catal., 1989, 115, 301 CrossRef CAS.
- T. Barakat, J. C. Rooke, E. Genty, R. Cousin, S. Siffert and B. L. Su, Energy Environ. Sci., 2013, 6, 371 CAS.
- A. Venugopal and M. S. Scurrell, Appl. Catal., A, 2004, 258, 241 CrossRef CAS PubMed.
- J. Qi, J. Chen, G. D. Li, S. X. Li, Y. Gao and Z. Y. Tang, Energy Environ. Sci., 2012, 5, 8937 CAS.
- C. Milone, M. L. Tropeano, G. Gulino, G. Neri, R. Ingoglia and S. Galvagno, Chem. Commun., 2002, 868 RSC.
- T. Yan, D. W. Redman, W. Y. Yu, D. W. Flaherty, J. A. Rodriguez and C. B. Mullins, J. Catal., 2012, 294, 216 CrossRef CAS PubMed.
- W. Gao, L. F. Ji, L. Li, G. W. Cui, K. H. Xu, P. Li and B. Tang, Biomaterials, 2012, 33, 3710 CrossRef CAS PubMed.
- K. Li, Y. J. Lai, W. Zhang and L. T. Jin, Talanta, 2011, 84, 607 CrossRef CAS PubMed.
- J. J. Wu, Y. L. Lee, H. H. Chiang and D. K. P. Wong, J. Phys. Chem. B, 2006, 110, 18109 Search PubMed.
- G. X. Wang, X. L. Gou, J. Horvat and J. Park, J. Phys. Chem. C, 2008, 112, 15220 CAS.
- N. Suong, T. Do, D. M. Schaetzl, B. Dey, A. C. Seabaugh and S. K. Fullerton-Shirey, J. Phys. Chem. C, 2012, 116, 21216 Search PubMed.
- E. V. Shevchenko, M. I. Bodnarchuk, M. V. Kovalenko, D. V. Talapin, R. K. Smith, S. Aloni, W. Heiss and A. P. Alivisatos, Adv. Mater., 2008, 20, 4323 CrossRef CAS.
- M. Spuch-Calvar, J. Perez-Juste and L. M. J. Liz-Marzan, Colloids Interface Sci. Ser., 2007, 310, 297 CrossRef CAS PubMed.
- Z. Zhong, J. Lin, S. P. Teh, J. Teo and F. M. Dautzenberg, Adv. Funct. Mater., 2007, 17, 1402 CrossRef CAS.
- Q. Y. Hao, S. Liu, X. M. Yin, Z. F Du, M. Zhang, L. M. Li, Y. G. Wang, T. H. Wang and Q. H. Li, CrystEngComm, 2011, 13, 806 RSC.
- J. Zhang, X. H. Liu, S. H. Wu, M. J. Xu, X. G. Guo and S. R. Wang, J. Mater. Chem., 2010, 20, 6453 RSC.
- A. M. Visco, F. Neri, G. Neri, A. Donato, C. Milone and S. Galvagno, Phys. Chem. Chem. Phys., 1999, 1, 2869 RSC.
- X. D. Ma, Q. Sun, X. Feng, X. He, J. Guo, H. W. Sun and H. Q. Cao, Appl. Catal., A, 2013, 450, 143 CrossRef CAS PubMed.
- X. D. Ma, J. S. Shen, W. Y. Pu, H. W. Sun, Q. Pang, J. Guo, T. Zhou and H. Q. Cao, Appl. Catal., A, 2013, 466, 68 CrossRef CAS PubMed.
- T. Kawabe, K. Tabata, F. Suzuki, Y. Yamaguchi and Y. Nagasawa, J. Phys. Chem. B, 2001, 105, 4239 CrossRef CAS.
- J. C. Dupin, D. Gonbeau, P. Vinatier and A. Levasseur, Phys. Chem. Chem. Phys., 2000, 2, 1319 RSC.
- L. Yu, H. B. Wu and X. W. Lou, Adv. Mater., 2013, 25, 2296 CrossRef CAS PubMed.
- X. D. Ma, X. Feng, X. He, H. W. Guo, L. Lv, J. Guo, H. Q. Cao and T. Zhou, Microporous Mesoporous Mater., 2012, 158, 214 CrossRef CAS PubMed.
- X. D. Ma, H. W. Sun, Q. Sun, X. Feng, H. W. Guo, B. Fan, S. Zhao, X. He and L. Lv, Catal. Commun., 2011, 12, 426 CrossRef CAS PubMed . Jun Song Chen.
- T. Zhu, X. H. Yang, H. G. Yang and X. W. Lou, J. Am. Chem. Soc., 2012, 134, 17388 CrossRef PubMed.
- Y. Gurbuz, W. P. Kang, J. L. Davidson and D. V. Kerns, Sens. Actuators, B, 2004, 99, 207 CrossRef CAS PubMed.
- J. Getino, L. Ares, J. I. Robla, M. C. Horrillo, I. Sayago, M. J. Fernandez, J. Rodrigo and J. Gutierrez, Sens. Actuators, B, 1999, 59, 249 CrossRef CAS.
- B. K. Min and C. M. Friend, Chem. Rev., 2007, 107, 2709 CrossRef CAS PubMed.
- M. C. Daniel and D. Astruc, Chem. Rev., 2004, 104, 293 CrossRef CAS PubMed.
- J. Zhang, X. H. Liu, S. H. Wu, M. J. Xu, X. Z. Guo and S. R. Wang, J. Mater. Chem., 2010, 20, 6453 RSC.
- X. L. Liu, J. Zhang, X. G. Guo, S. H. Wu and S. R. Wang, Nanoscale, 2010, 2, 1178 RSC.
- X. H. Liu, J. Zhang, L. W. Wang, T. L. Yang, X. Z. Guo, S. H. Wu and S. R. Wang, J. Mater. Chem., 2011, 21, 349 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c3ra44779k |
| This journal is © The Royal Society of Chemistry 2014 |