Composite of graphene quantum dots and Fe3O4 nanoparticles: peroxidase activity and application in phenolic compound removal†
Abstract
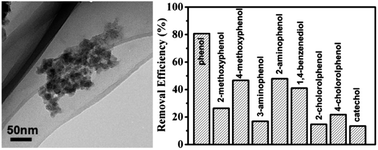
Graphene quantum dots (GQDs) are graphene sheets with lateral sizes less than 100 nm, and have a higher electron conjugate state and a better dispersion ability in aqueous solution compared to micrometer-sized graphene oxide (GO) sheets. Therefore they can overcome the drawbacks of GO and are an ideal candidate for nano-composites. In this work, composites of GQDs and Fe3O4 nanoparticles (NPs) (GQDs/Fe3O4) were prepared via a one-step co-precipitation approach. The as-prepared GQDs/Fe3O4 composites showed superb peroxidase-like activities, which were much higher than composites of micrometer sized GO and Fe3O4 NPs (GO/Fe3O4), individual GQDs, and individual Fe3O4 NPs. The excellent peroxidase activities of the GQDs/Fe3O4 composites can be attributed to the unique properties of GQDs and the synergistic interactions between the GQDs and Fe3O4 NPs. The GQDs/Fe3O4 composites also exhibited a higher stability and reusability than natural peroxidases. The application of a GQDs/Fe3O4 composite as a catalyst for the removal of phenolic compounds from aqueous solutions was explored with nine phenolic compounds, and showed better or comparable removal efficiencies for some phenolic compounds compared to native horseradish peroxidase (HRP) under the same conditions. The extraordinary catalytic performance and physical properties of the as-prepared GQDs/Fe3O4 composite render it practically useful for industrial wastewater treatment.

 Please wait while we load your content...
Please wait while we load your content...