A novel aluminium–air secondary battery with long-term stability
Abstract
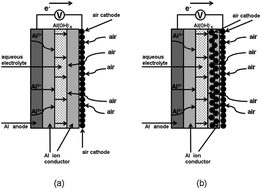
An aluminium–air secondary battery was fabricated by using an aluminium ion conductor, Al2(WO4)3, and plain salt water as an electrolyte. The battery functioned for approximately one month. It was also possible to enhance the cell capacity and suppress the yield of by-products, by modifying the cell structure.

 Please wait while we load your content...
Please wait while we load your content...