Cellulose-based catalytic membranes fabricated by deposition of gold nanoparticles on natural cellulose nanofibres†
Tao Niu,
Junbo Xu,
Wei Xiao and
Jianguo Huang*
Department of Chemistry, Zhejiang University, Hangzhou, Zhejiang 310027, China. E-mail: jghuang@zju.edu.cn; Fax: +86 571 8795 1202
First published on 10th December 2013
Abstract
Deposition of gold nanoparticles on titania gel film pre-coated cellulose nanofibres of filter paper yields bulk cellulose-based catalytic membranes, which exhibit excellent catalytic activities toward the reduction of 4-nitrophenol to 4-aminophenol through a facile filtration process.
Metallic nanoparticles have attracted growing interest from the catalysis research community due to their high surface-to-volume ratio and distinctive surface electronic properties,1 which lead to the excellent catalytic performances in various chemical processes.2 In particular, gold nanoparticles (Au-NPs), are regarded as some of the most attractive catalysts.2a,3 However, on account of the small sizes and high surface energy of Au-NPs, direct introduction of them in the reaction systems readily results in aggregation, which dramatically lowers their catalytic activities;2b,3e on the other hand, sufficient separation and recycling of the Au-NPs from the reaction mixtures are generally difficult.2b To overcome such drawbacks, immobilization or dispersion of Au-NPs in various solid matrices like metal oxides and polymeric materials has been developed.4 And free-standing porous membranes were employed as promising supports for the immobilization of Au-NPs for better catalytic performances.4a–c,5 However, the practical applications are limited due to the complicated preparative processes to make such membranes.
Cellulose substances, such as ordinary commercial filter paper, are abundant and low-cost materials, possessing the intrinsic unique features of high porosity, good flexibility, robust mechanical strength as well as chemical stability. It can be an ideal choice to employ filter paper as a candidate support for the immobilization of Au-NPs. We have pioneered deposition of ultrathin metal oxide gel films on the cellulose nanofibres of filter paper by means of a surface sol–gel approach,6 and subsequently achieved the functional surface modification through self-assembly of specific guest substances.7 Herein, by introduction of Au-NPs on the titania gel film pre-coated cellulose nanofibres of bulk filter paper, Au-NP-containing cellulose-based membranes were fabricated. Unlike the powder-like catalysts, such cellulose-based catalytic membranes were able to fulfill sufficient catalytic reduction of 4-nitrophenol (4-NP) to 4-aminophenol (4-AP) through simple filtration of the aqueous solution of the reaction mixture (Scheme 1), exhibiting exceptional catalytic activities.
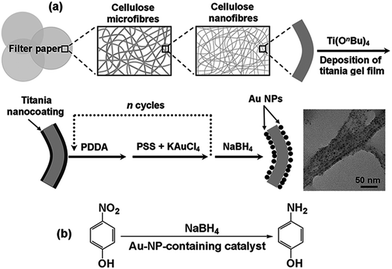 | ||
| Scheme 1 (a) Schematic representation of the fabrication of the catalytic cellulose/Au-NP composite membranes. (b) The model reaction used to investigate the catalytic performance of the membranes. | ||
To fabricate the cellulose-based catalytic membranes, 5-cycle deposition of titania gel layer was first performed to form a uniform titania film coated on the cellulose nanofibers of bulk filter paper (Scheme 1a, ESI†), the sample is denoted as cellulose/(TiO2)5. Afterwards, positively charged poly(diallyl-dimethylammonium chloride) (PDDA) layer as well as negatively charged two-component layer containing poly(sodium-p-styrenesulfonate) (PSS) and KAuCl4 were sequentially alterna-tively deposited onto the titania gel film surface for n times (Scheme 1a, ESI†). Followed by treatment with aqueous NaBH4 solution, the anchored KAuCl4 was reduced to Au-NPs (eqn (1)), yielding the Au-NP-containing cellulose-based membranes (denoted as cellulose/(TiO2)5/Au@(PDDA/PSS)n).
| KAuCl4 + NaBH4 + H2O → Au + B(OH)3 + NaCl + KCl + HCl | (1) |
The as-fabricated cellulose/(TiO2)5 composite sheet displayed the initial white colour as the pure filter paper, while the Au-NP containing composite membranes showed light pink colour (Fig. 1a), which is indicative of the formation of Au-NPs on the bulk filter paper support.8 Fig. 1b shows the solid UV-vis spectra of the surface-modified cellulose materials. The cellulose/(TiO2)5 paper gave only one absorption band at 256 nm, ascribed to the absorption of nanometer-thick titania film.7a,9 In addition to the absorption band of titania, the cellulose-based catalytic membrane (i.e., the cellulose/(TiO2)5/Au@(PDDA/PSS)10 paper) showed two new absorption bands at 520 nm and 228 nm. The former is due to the surface plasmon resonance (SPR) absorption of the deposited Au-NPs;8a,10 and the later is attributed to the π → π* electronic transitions of the benzene ring in the deposited PSS molecules as well as the n → σ* electronic transitions of pyrrole structure in the deposited PDDA molecules.11
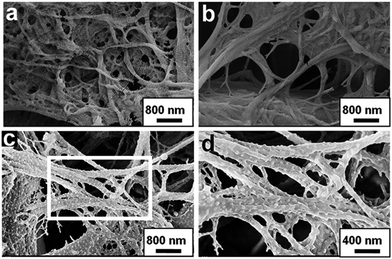
Fig. 2a presents a field emission scanning electron microscopy (FE-SEM) image of the pure filter paper used, showing hierarchically fibrous network structures. Deposition of the titania gel films on the cellulose nanofibres resulted in no obvious change on the nanoscopic morphologies (Fig. 2b). According to our previous work, further deposition of PDDA/PSS multilayers onto the titania gel film pre-coated cellulose nanofibres gave uniform and rather smooth coating of the fibres without any particle structures as revealed by FE-SEM observation.12 However, remarkable morphological difference on nanometer scales was observed for the current cellulose-based catalytic membranes. As can be clearly seen from Fig. 2c and d, lots of nanoparticles with the diameters of ∼50 nm were homogeneously distributed on the composite nanofibres of the cellulose/(TiO2)5/Au@(PDDA/PSS)10 paper, although the initial hierarchically fibrous structure of filter paper was retained. The transmission electron microscopy (TEM) observation of the composite nanofibres isolated from the cellulose/(TiO2)5/Au@(PDDA/PSS)10 paper revealed that uniform gold nanoparticles with narrow size distribution were attached on the composite fibres (Fig. 3), the mean size of which is 3.5 nm with a standard deviation of 0.7 nm (Fig. S1, ESI†). This result agrees well with the Au-NP SPR band shown above in the UV-vis spectrum of the sample (Fig. 1, blue curve).8a As for the sample cellulose/(TiO2)5/Au@(PDDA/PSS)20 paper, similar electron microscopic observation results were obtained (Fig. S2, ESI†). It is needed to be noted that the remarkable size difference of the particles in between the FE-SEM and TEM observations is due to the formation of Au@polyelectrolyte core–shell structures, where the gold nanoparticles were capped with the PDDA/PSS layers. In the FE-SEM measurement, the whole core–shell structured particles were observed; while in the TEM survey, the polyelectrolyte shells of the composite particles were invisible due to the lack of contrast between the core gold nanoparticles and the background. Similar results were reported by F. Caruso and co-workers in the preparation of the PDADMAC/PSS-coated carboxylate-modified gold nanoparticles.13 For the current case, each large-particle shown in the SEM images may contain a number of gold nanoparticles inside, and other gold nanoparticles exist in the space in between the large-particles which are also coated with the polyelectrolyte layers.
The catalytic activities of the cellulose-based catalytic membranes were evaluated employing the reduction reaction of 4-nitrophenol (4-NP) illustrated in Scheme 1b. In this reaction, the reduction of 4-NP by NaBH4 to 4-aminophenol (4-AP) was catalyzed by the cellulose/Au-NP composite and the process of which was monitored by UV-vis spectrometry. The original aqueous solution of 4-NP exhibited a characteristic peak centred at 317 nm, while it shifted to 400 nm upon addition of NaBH4 due to the formation of 4-nitrophenolate ions (Fig. S3, ESI†),4a,8a and the resultant solution displayed yellow in colour. Interestingly, by simply filtering the aqueous feed solution of the reaction mixture containing 4-NP (0.1 mM) and excess NaBH4 (10 mM) through the cellulose-based catalytic membranes, the peak at 400 nm for the filtrate decreased drastically compared with that for the initial feed solution (Fig. 4). Meanwhile, a new peak appeared at 298 nm in the UV-vis spectra of the filtrate, which was assigned to the corresponding product 4-AP.4a,8a The conversion ratio of 4-NP was as high as 74% by one-cycle filtration of 10 mL feed solution through the cellulose/(TiO2)5/Au@(PDDA/PSS)10 paper, which was further promoted to 94.3% and 98.7% after filtering the feed solution for double and triple cycles, respectively (Fig. 4a). The original yellow feed solution faded to colourless after three-cycles of filtration, agreeing well with the sufficient reduction of 4-NP (Fig. 4a). For the cellulose/(TiO2)5/Au@(PDDA/PSS)20 paper, surprisingly, the conversion ratio of 4-NP reached up to 95.3% by single filtration of the above-mentioned feed solution, and complete reduction of 4-NP to 4-AP was almost accomplished after double filtrations with the conversion ratio over 99.5% (Fig. 4b). In sharp contrast, after filtering 10 mL aqueous feed solution through pure filter paper, the titania gel film coated paper (i.e., the cellulose/(TiO2)5 paper) or titania gel film and PDDA/PSS multilayer modified paper (i.e., the cellulose/(TiO2)5/(PDDA/PSS)10 paper) for three times, no catalytic activity was detected (Fig. S4, ESI†). Consequently, the extraordinary catalytic activities of the currently developed cellulose-based catalytic membranes were resulted from the deposited Au-NPs. As measured by ICP-MS, the Au content in the cellulose/(TiO2)5/Au@(PDDA/PSS)10 paper and the cellulose/(TiO2)5/Au@(PDDA/PSS)20 paper was 0.18% and 0.2%, respectively, indicating higher Au content leads to better catalytic performance.
In previous reports, the formation of Au-NPs on crystalline cellulose nanofibres or cellulose powder and adsorption of pre-synthesized Au-NPs on nanocrystalline cellulose gave some catalytic cellulose/Au-NP composite materials.8a,10,14 However, these materials are not in the bulk form, resulting in inconvenience in the recycling of the catalysts. And, citrate-stabilized Au colloids were reported to be assembled into porous alumina or polycarbonate membranes, allowing the flow-through catalytic reduction of 4-NP,4a,b but an affiliated peristaltic pump or pressurizing apparatus has to be applied. Besides, Au-NPs were deposited on carbonaceous nanofibres (CNF) to produce a bulk porous membrane capable of catalyzing the reduction of 4-NP through filtration of the reaction mixture,4c however, the CNFs were prepared using ultrathin Te nanowires as template, which is a quite limited source.4c,15 For the catalytic system developed herein, the functional surface modification of cellulose nanofibres of filter paper with Au-NP is facile and controllable; the present catalytic process is just a filtration process, which is very simple but quite effective; filter paper is an abundant and inexpensive material, hence facilitating the fabrication of the bulk cellulose-based catalytic membranes on a large scale.
In conclusion, bulk cellulose-based catalytic membranes were fabricated by deposition of Au-NPs on titania gel film pre-coated cellulose nanofibres of filter paper, which exhibited splendid catalytic activities toward the reduction of 4-NP to 4-AP through a facile filtration process. Biomimetic synthesis has been proven to be a unique pathway to functional materials with designed properties,16 it is believed that the methodology presented herein can be expanded to a broad range of noble metallic nanoparticles, and the resultant hybrid materials are expected to catalyze more types of momentous reactions.
This work was supported by the 973 program of China (2009CB930104) and NSFC (2117392, J1210042).
References
- D. Astruc, F. Lu and J. R. Aranzaes, Angew. Chem., Int. Ed., 2005, 44, 7852–7872 CrossRef CAS PubMed.
- (a) A. S. K. Hashmi and G. J. Hutchings, Angew. Chem., Int. Ed., 2006, 45, 7896–7936 CrossRef PubMed; (b) A. Z. Moshfegh, J. Phys. D: Appl. Phys., 2009, 42, 233001 CrossRef; (c) M. Stratakis and H. Garcia, Chem. Rev., 2012, 112, 4469–4506 CrossRef CAS PubMed.
- (a) N. Ta, J. Liu, S. Chenna, P. A. Crozier, Y. Li, A. Chen and W. Shen, J. Am. Chem. Soc., 2012, 134, 20585–20588 CrossRef CAS PubMed; (b) M. Turner, V. B. Golovko, O. P. H. Vaughan, P. Abdulkin, A. Berenguer-Murcia, M. S. Tikhov, B. F. G. Johnson and R. M. Lambert, Nature, 2008, 454, 981–984 CrossRef CAS PubMed; (c) M. Okumura, T. Akita and M. Haruta, Catal. Today, 2002, 74, 265–269 CrossRef CAS; (d) A. Primo, T. Marino, A. Corma, R. Molinari and H. García, J. Am. Chem. Soc., 2011, 133, 6930–6933 CrossRef CAS PubMed; (e) Q. Zhang, T. Zhang, J. Ge and Y. Yin, Nano Lett., 2008, 8, 2867–2871 CrossRef CAS PubMed.
- (a) D. M. Dotzauer, J. Dai, L. Sun and M. L. Bruening, Nano Lett., 2006, 6, 2268–2272 CrossRef CAS PubMed; (b) D. M. Dotzauer, S. Bhattacharjee, Y. Wen and M. L. Bruening, Langmuir, 2009, 25, 1865–1871 CrossRef CAS PubMed; (c) H.-W. Liang, W.-J. Zhang, Y.-N. Ma, X. Cao, Q.-F. Guan and W.-P. Xu, ACS Nano, 2011, 5, 8148–8161 CrossRef CAS PubMed; (d) L. You, Y. Mao and J. Ge, J. Phys. Chem. C, 2012, 116, 10753–10759 CrossRef CAS; (e) G. Marcelo, A. Muñoz-Bonilla and M. Fernández-Garcia, J. Phys. Chem. C, 2012, 116, 24717–24725 CrossRef CAS.
- A. Julbe, D. Farrusseng and C. Guizard, J. Membr. Sci., 2001, 181, 3–20 CrossRef CAS.
- J. Huang and T. Kunitake, J. Am. Chem. Soc., 2003, 125, 11834–11835 CrossRef CAS PubMed.
- (a) T. Niu, Y. Gu and J. Huang, J. Mater. Chem., 2011, 21, 651–656 RSC; (b) W. Xiao and J. Huang, Langmuir, 2011, 27, 12284–12288 CrossRef CAS PubMed.
- (a) H. Koga, E. Tokunaga, M. Hidaka, Y. Umemura, T. Saito, A. Isogai and T. Kitaoka, Chem. Commun., 2010, 46, 8567–8569 RSC; (b) F. Xu, H. Ma, S. Xiao, M. Shen, R. Guo, X. Cao and X. Shi, J. Mater. Chem., 2011, 21, 4493–4501 RSC.
- J. Huang, I. Ichinose, T. Kunitake and A. Nakao, Langmuir, 2002, 18, 9048–9053 CrossRef CAS.
- E. Lam, S. Hrapovic, E. Majid, J. H. Chong and J. H. T. Luong, Nanoscale, 2012, 4, 997–1002 RSC.
- (a) Q. Wang and P. J. Hauser, Cellulose, 2009, 16, 1123–1131 CrossRef CAS; (b) Y. Xia and J. Ouyang, ACS Appl. Mater. Interfaces, 2010, 2, 474–483 CrossRef CAS PubMed.
- Y. Gu, T. Niu and J. Huang, J. Mater. Chem., 2010, 20, 10217–10223 RSC.
- K. S. Mayya, B. Schoeler and F. Caruso, Adv. Funct. Mater., 2003, 13, 183–188 CrossRef CAS.
- T. Ishida, H. Watanabe, T. Bebeko, T. Akita and M. Haruta, Appl. Catal., A, 2010, 377, 42–46 CrossRef CAS PubMed.
- H.-W. Liang, L. Wang, P.-Y. Chen, H.-T. Lin, L.-F. Chen, D. He and S.-H. Yu, Adv. Mater., 2010, 22, 4691–4695 CrossRef CAS PubMed.
- (a) J. Li, H. Möhwald, Z. An and G. Lu, Soft Matter, 2005, 1, 259–264 RSC; (b) Q. He, Y. Cui and J. Li, Chem. Soc. Rev., 2009, 38, 2292–2303 RSC; (c) Z. An, H. Möhwald and J. Li, Biomacromolecules, 2006, 7, 580–585 CrossRef CAS PubMed; (d) Q. He, H. Möhwald and J. Li, Macromol. Rapid Commun., 2009, 30, 1538–1542 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details, electron microscopy images and UV-vis spectra. See DOI: 10.1039/c3ra44622k |
| This journal is © The Royal Society of Chemistry 2014 |