Synthesis, crystal structures and luminescent properties of CdII and ZnII complexes assembled by 4-aminophenylhydroxamic acid†
Yanmei
Chen
a,
Qian
Gao
a,
Yonglu
Liu
a,
Yanyuan
Cao
a,
Dandan
Gao
b,
Jinna
Liu
a,
Jingjing
Zhao
a,
Yahong
Li
*ac,
Wei
Liu
*a and
Wu
Li
b
aKey Laboratory of Organic Synthesis of Jiangsu Province, College of Chemistry, Chemical Engineering and Materials Science, Soochow University, Suzhou 215123, China. E-mail: liyahong@suda.edu.cn
bQinghai Institute of Salt Lakes, Chinese Academy of Sciences, Xining 810008, China
cState Key Laboratory of Applied Organic Chemistry, Lanzhou University, Lanzhou 730000, China
First published on 4th November 2013
Abstract
Four complexes of composition [Cd(4-Apha)2]n (1), [Zn2(4-Apha)4(H2O)2]·2H2O (2), [Zn(4-Apha)(CH3COO)]n (3), and [Zn4(4-Apha)4(CH3COO)4(H2O)]n·2nCH3OH (4) (4-AphaH = 4-aminophenylhydroxamic acid) have been synthesized in different solvents and characterized by X-ray diffraction, IR spectroscopy, thermal analysis and elemental analysis. X-ray crystal analysis reveals that these complexes exhibit four varieties of structures. Complex 1 displays a 1-D chain structure, and complex 2 is binuclear. Complexes 3 and 4 possess different 3-D structures. Fluorescence emissions are observed for complexes 1, 2 and 4 in solid state.
Introduction
Hydroxamic acids (HAc) are important bioligands, broadly utilized in medicine and chemical biology.1 They function as potent and selective inhibitors of enzymes and have hypotensive, anti-cancer, anti-malarial and anti-fungal properties.2 They could coordinate to metals in monodentate, bidentate and tridentate fashion, as observed in some urease and metal-proteins.3 The powerful metal-chelating ability and rich coordination modes of hydroxamic acids have also been employed to construct a diverse host of fascinating coordination compounds including classical homometallic di- and trinuclear complexes,4 high-nuclear clusters,5 and transition- and lanthanide-transition- metallacrowns.6 It is found that the coordination behaviours of phenylhydroxamic acids can be tailored by the incorporation of various functional groups, e.g., –NMe2,5b –OH,7 –NH2,8etc., at ortho, meta and (or) para positions of benzene rings. However, to our surprise, hydroxymic acids have rarely been utilized as building blocks in creating 1-D, 2-D and 3-D coordination polymers,9 which might have potential applications in single-chain magnets, gas adsorption, and luminescence materials.10We are interested in employing 4-aminophenylhydroxamic acid (4-AphaH) as a ligand to create the coordination polymers of CdII and ZnII. In 4-AphaH, the hydroxamic group can assemble several metals into a polynuclear moiety, and the extra amine group in para position may serve as the bridging ligand to link the polynuclear moiety with another metal, generating coordination polymers. Furthermore, the chemistry of diamagnetic ZnII and CdII coordination complexes in the forms of monomeric, multinuclear, and polymeric species have attracted much attention in recent years.11 Coordination polymers of CdII and ZnII have been widely investigated for photoluminescent properties and for potential applications as fluorescence-emitting materials, such as light-emitting diodes (LEDs).12 They have high thermal stability and the ability to affect the emission wavelength of the organic material by metal coordination. For applications in electroptical devices, ZnII complexes supported by 2-benzothiazol-2-yl-phenol13 or 1H-pyrrolo[2,3-b]pyridine ligands,14 are electroluminescent materials and have been applied in organic light-emitting diodes (OLEDs). With coordination polymeric structures,15 the inhibition of peptide deformylase of ZnII complexes in biological systems,16 the catalytic activity of CdII complexes in cyanosilylation reactions17 have also been studied, whereas the coordination polymers of ZnII/CdII of hydroxamic acids were rarely reported in the literature.16b,9b,18
The results of the present investigation gave four kinds of complexes: [Cd(4-Apha)2]n (1), [Zn2(4-Apha)4(H2O)2]·2H2O (2), [Zn(4-Apha)(CH3COO)]n (3), and [Zn4(4-Apha)4(CH3COO)4(H2O)]n·2nCH3OH (4) (4-AphaH = 4-aminophenylhydroxamic acid). Herein, we report the syntheses, structures, thermal stabilities and luminescent properties of these complexes.
Experimental section
General procedure
Syntheses of the complexes
The PXRD patterns of complexes 1–4 are presented in Fig. S5.† The diffraction peaks of both simulated and experimental patterns match well, indicating the phase purities of these complexes.
X-ray crystallography
Data were collected at room temperature on Bruker Smart ApexII diffractometer equipped with a graphite monochromator utilising Mo Kα radiation (λ = 0.71073 Å); the ω and φ scan technique was applied. The structures were solved by direct methods using SHELXS-97![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 19 and refined on F2 using full-matrix least-squares with SHELXL-97.20 Crystallographic data together with refinement details for the new complexes reported in this work are summarised in Table 1. See CIF files for further details.
19 and refined on F2 using full-matrix least-squares with SHELXL-97.20 Crystallographic data together with refinement details for the new complexes reported in this work are summarised in Table 1. See CIF files for further details.
| 1 | 2 | 3 | 4 | |
|---|---|---|---|---|
| a Including solvate molecules. b Mo Kα radiation. c R 1 = Σ(|Fo| − |Fc|)/Σ(|Fo|) for observed reflections. d w = 1/[σ2(Fo2) + (αP)2 + bP] and P = [max(Fo2, 0) + 2Fc2]/3. e wR2 = {Σ[w(Fo2 − Fc2)2]/Σ[w(Fo2)2]}1/2 for all data. | ||||
| Formulaa | CdC14H14N4O4 | Zn2C28H36N8O12 | ZnC9H10N2O4 | Zn4C38H50N8O19 |
| M/g mol−1a | 414.70 | 807.39 | 275.56 | 1184.34 |
| T/K | 223(2) | 296(2) | 296(2) | 290(2) |
| λ b/Å | 0.71073 | 0.71073 | 0.71073 | 0.71073 |
| Crystal system | Orthorhombic | Monoclinic | Tetragonal | Monoclinic |
| Space group | Iba2 | P21/n | I41/a | Cc |
| a/Å | 12.121(2) | 10.5931(6) | 19.7107(9) | 26.7007(19) |
| b/Å | 17.543(4) | 7.6567(4) | 19.7107(9) | 9.7429(6) |
| c/Å | 6.9785(14) | 19.8339(11) | 11.0593(10) | 18.8038(12) |
| β/° | 90 | 93.2620(10) | 90 | 90.254(2) |
| V/Å3 | 1483.9(5) | 1606.09(15) | 4296.7(5) | 4891.6(6) |
| Z | 4 | 2 | 16 | 4 |
| ρ c/g cm−3 | 1.856 | 1.670 | 1.704 | 1.608 |
| μ/mm−1 | 1.498 | 1.570 | 2.286 | 2.018 |
| F(000) | 824 | 832 | 2240 | 2424 |
| θ range/° | 2.04–24.99 | 2.06–27.95 | 2.07 to 26.35 | 2.17–28.31 |
| Measd/independent | 3194/1291 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 613/3828 613/3828 |
26![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 488/2199 488/2199 |
34![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 256/11 256/11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 775 775 |
| R int reflections | 0.0243 | 0.0202 | 0.0233 | 0.0252 |
| Obsd reflns [I > 2σ (I)] | 1291 | 3828 | 2199 | 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 775 775 |
| GOF on F2 | 1.066 | 1.063 | 1.133 | 1.093 |
| R 1 c | 0.0183 | 0.0287 | 0.0340 | 0.0385 |
| wR2d,e | 0.0458 | 0.0795 | 0.1082 | 0.1165 |
| (Δρ)max,min/e Å−3 | 0.212, −0.215 | 0.814, −0.443 | 1.002, −0.288 | 0.944, −0.502 |
Results and discussion
Syntheses of the complexes
Complex 1 was prepared by heating a mixture of Cd(CH3COO)2·2H2O, 4-AphaH and water under soft-hydrothermal conditions at 70 °C. Pink rod crystals of [Cd(4-Apha)2]n (1) were generated after heating the mixture for 1 day. We also explored the reactions of Cd(CH3COO)2·2H2O and 4-AphaH in methanol and ethanol, respectively, and the same crystals were afforded.Treatment of Zn(CH3COO)2·2H2O with 4-AphaH in water gave a dinuclear complex [Zn2(4-Apha)4(H2O)2]·2H2O (2). When the reaction of Zn(CH3COO)2·2H2O and 4-AphaH was conducted in ethanol, a coordination polymer [Zn(4-Apha)(CH3COO)]n (3) was produced.
Intrigued by the observation that the complexes with the totally different compositions were afforded in the different solvents, we examined the reaction of Zn(CH3COO)2·2H2O and 4-AphaH in methanol. Complex [Zn(4-Apha)(CH3COO)]n (3) was generated. We were curious if another new complex could be given in the mixed solvent of water and methanol, we then investigated the reaction of Zn(CH3COO)2·2H2O and 4-AphaH in the mixture of water and methanol (v/v = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9). Gratifyingly, a coordination polymer [Zn4(4-Apha)4(CH3COO)4(H2O)]n·2nCH3OH (4) was prepared.
9). Gratifyingly, a coordination polymer [Zn4(4-Apha)4(CH3COO)4(H2O)]n·2nCH3OH (4) was prepared.
The successful generation of 2–4 indicates that the coordination complexes with different structures could be assembled via employing different solvents, demonstrating the synthetic novelty of this work.
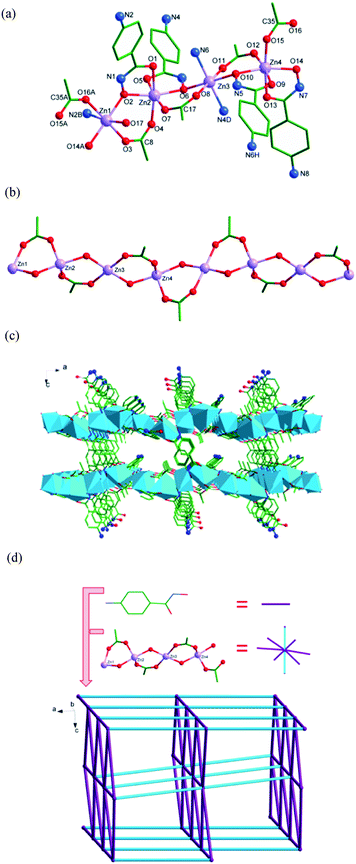
Description of structures
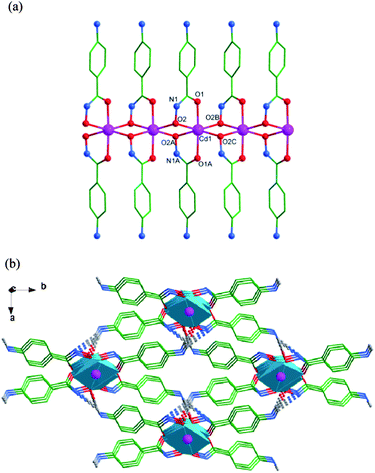
Complex 1 is a member of a small family of chain complexes with hydroxamic acids as ligands.1,11b The chain configuration is a common topology in CdII compounds,12a but it is never observed in the coordination complexes of hydroxamic acids.
The intermolecular hydrogen interactions between N1 atoms of the NH units as hydrogen donors and N2 atoms of NH2 groups as hydrogen acceptors of the 4-Apha− ligands (N1–H1⋯N2: the angle of N1–H1⋯N2 is 152.2°, the distance of H1⋯N2 is 2.27 Å, symmetry operation: −x + 1/2, y + 1/2, −z − 1/2) connect the binuclear complexes to generate a 2-D network structure in bc plane. The hydrogen bonds between N (HN and NH2) atoms of the 4-Apha− ligands as hydrogen donors and O atoms of water molecules as hydrogen acceptors connect the 2-D networks giving a 3-D supramolecular structure (Fig. 2b). Lattice water molecules are connected to the main structure by O–H⋯O hydrogen bonds. See Table S4† for the details.
To better understand the nature of the 3-D framework, a topological approach has been applied. Each Zn1 atom can be considered as a 3-connected node, and each 4-Apha− ligand serving as a linear linker connects two adjacent 3-connected nodes (Fig. 3d). The whole structure can be simplified to a topology with Schläfli symbol of 82.10.21
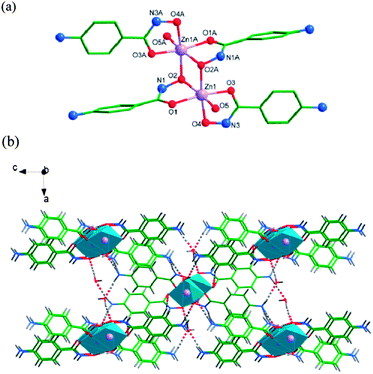
In 3, the N1–H1⋯O5 interaction between N1 atom of 4-Apha− ligand as the donor and O5 atom as the acceptor, with the N1–H1⋯O5 angle of 124.8°, the H1⋯O5 distance of 2.20 Å, symmetry operation y − 1/4, x + 3/4, z − 1/4 (Table S6†), occurs. A weak π⋯π interaction between the benzene rings of the 4-Apha− ligands has been found, with the π-centroid to π-centroid distance of 3.836(2) Å.
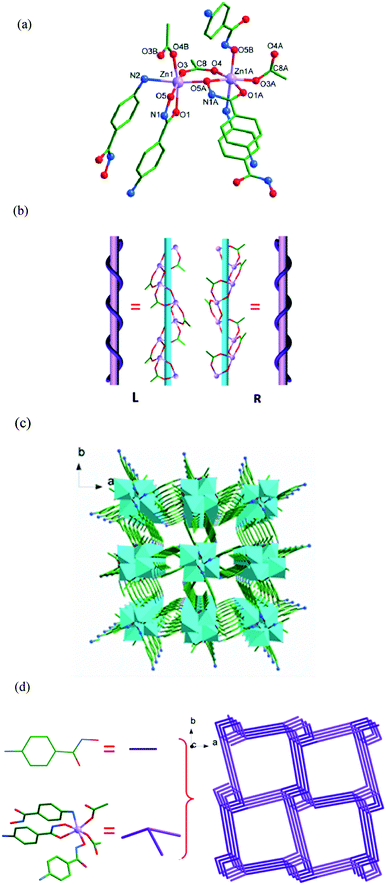
To better understand the nature of the 3-D framework, a topological approach has been applied. The tetranuclear [ZnO(CH3COO)]4 unit (Zn1, Zn2, Zn3 and Zn4 as the node centres) can be considered as a 8-connected node, and each 4-Apha− serving as a linear linker connects two adjacent [ZnO(CH3COO)]4 units (Fig. 4d). The whole structure can be simplified to a topology with Schläfli symbol of 36·414·57·6.21
Both intermolecular and intramolecular hydrogen interactions between N atoms (NH and NH2) of 4-Apha− as hydrogen donors and O atoms of 4-Apha− (or acetic acid, or coordinated water molecular) as the acceptors have been found in 4. Interactions between O17 atom of coordination water molecule as the donor and N8 (or O7) atom of the 4-Apha− ligand has been found (O17–H17A⋯N8: the O17–H17A⋯N8 angle is 165.4°, the H17A-N8 distance is 2.03 Å, and the symmetry operation −1/2 + x, −1/2 + y, z; O17H–17B⋯O7: the angle of O17H–17B⋯O7 is 102.0°, the distance of 17B⋯O7 is 2.49 Å, and no symmetry operation). These hydrogen bonds enhance the stability of the crystal structure. The lattice methanol molecules connect the main structure by O19–H19⋯N6 and O18–H18⋯O5 hydrogen interactions (O19–H19⋯N6: the distance of H19⋯N6 is 2.51 Å, and the angle of O19–H19⋯N6 is 123.3°; O18–H18⋯O5: the angle of O18–H18⋯O5 is 164.2°, the distance of H18⋯O5 is 2.05 Å. both of them have no symmetry operation). See Table S8† for the details.
Complexes 3 and 4 join a small family of 3-D frameworks with hydroxamic acids as ligands.1,9b However, the ZnII complexes with 3-D structures have never been observed in the coordination complexes of hydroxamic acids.
Luminescence properties
The luminescent properties of the 4-AphaH ligand and complexes 1 to 4 were investigated in the solid state at room temperature (Fig. 5 and S6†). Excited at 354 nm, the 4-AphaH ligand shows weak photoluminescence emission at 425 nm. Upon excitation at 368 nm, complex 1 exhibits a red-shift of 43 nm compared with the 4-AphaH ligand, with the maximum peaks at 468 nm. However, excited at 370 nm, complex 2 shows red-shift of 39 nm compared with the 4-AphaH ligand, with the maximum peaks at 464 nm. No luminescent property was observed for complex 3. Upon excitation at 364 nm, complex 4 exhibits a red-shift of 31 nm compare with the 4-AphaH ligand, with the maximum peaks at 456 nm. Compared with 4-AphaH, the red-shifted emission of 1, 2 and 4 may be ascribed as the intraligand (π–π*) fluorescent emission because similar emissions were observed for the 4-AphaH ligand at 428 nm.12,22 The pronounced fluorescence emissions of these complexes indicate their potential applications for photoactive materials.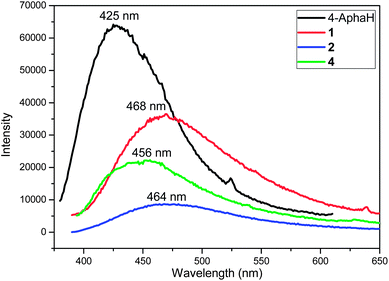 | ||
| Fig. 5 Room temperature emission spectra for 4-AphaH, 1, 2 and 4 in the solid state (emission slit 1 nm). | ||
Thermal analysis
The thermal stability of 1–4 was investigated using thermogravimetric analysis. As can be seen from Fig. S7–10,† the first weight loss of 2 took place between 92.0 °C and 115.4 °C (9.09%), which corresponds to the loss of all lattice and coordinated water molecules (calculated: 8.92%). Above 182.4 °C, the structure began to collapse and decompose. The first weight loss of 4 took place between 57.8 °C and 97.5 °C (6.89%), which corresponds to the loss of all lattice methanol molecules and coordinated water molecules (calculated 6.92%). Above 172.9 °C, the structure began to collapse and decompose. Complexes 1 and 3 were collapsed and decomposed from 213.5 °C and 196.3 °C, respectively.Conclusions
In summary, the CdII and ZnII coordination complexes based on 4-aminophenylhydroxamic acid (4-AphaH) were reported. One 1-D CdII chain complex, one dinuclear ZnII compound, and two ZnII coordination polymers with different 3-D network structures have been prepared and characterized. The solvents played vital roles in the control of the dimensionality of 2–4, as well as the crystal packing structures of 2 and 4. The strategy of rational assembly of the structures via choosing different solvents provides an efficient approach to ZnII coordination polymers. The luminescent properties of the free ligand, 1, 2 and 4 have been determined; the pronounced fluorescence emissions of 1, 2 and 4 reveal their potential applications for photoactive materials.Acknowledgements
The authors appreciate the financial support from Natural Science Foundation of China (21272167 and 21201127), A Project Funded by the Priority Academic Program Development of Jiangsu Higher Education Institution, Graduate Education Innovation Project in Jiangsu Province (CXZZ12_0808), and Qinghai Science & Technology Department of China (2011-G-208 and 2011-Z-722).References
- R. Codd, Coord. Chem. Rev., 2008, 252, 1387 CrossRef CAS PubMed.
- (a) C. J. Marmion, D. Griffith and K. B. Nolan, Eur. J. Inorg. Chem., 2004, 15, 3003 CrossRef; (b) C. J. Marmion, T. Murphy, J. R. Docherty and K. B. Nolan, Chem. Commun., 2000, 1153 RSC; (c) M. J. Miller, Chem. Rev., 1989, 89, 1563 CrossRef CAS.
- (a) M. A. Pearson, L. O. Nickel, R. P. Hausinger and P. A. Karplus, Biochemistry, 1997, 36, 8164 CrossRef CAS PubMed; (b) A. J. Stemmler, J. W. Kampf, M. L. Kirk and V. L. Pecoraro, J. Am. Chem. Soc., 1995, 117, 6368 CrossRef CAS; (c) S. Ciurli, S. Benini, W. R. Rypniewski, K. S. Wilson, S. Miletti and S. Mangani, Coord. Chem. Rev., 1999, 331, 190 Search PubMed.
- (a) I. M. Rio-Echevarria, F. J. White, E. K. Brechin, P. A. Tasker and S. G. Harris, Chem. Commun., 2008, 4570 RSC; (b) Z. Tomkowicz, S. Ostrovsky, H. Müller-Bunz, A. J. Hussein Eltmimi, M. Rams, D. A. Brown and W. Haase, Inorg. Chem., 2008, 47, 6956 CrossRef CAS PubMed.
- (a) Y. Cao, Y. Chen, L. Li, D. Gao, W. Liu, H. Hu, W. Li and Y. Li, Dalton Trans., 2013, 42, 10912 RSC; (b) D. Gaynor, Z. A. Starikova, S. Ostrovsky, W. Haase and K. B. Nolan, Chem. Commun., 2002, 506 RSC; (c) J. A. Johnson, J. W. Kampf and V. L. Pecoraro, Angew. Chem., Int. Ed., 2003, 42, 546 CrossRef CAS PubMed; (d) C. McDonald, T. Whyte, S. M. Taylor, S. Sanz, E. K. Brechin, D. Gaynor and L. F. Jones, CrystEngComm, 2013, 15, 6672 RSC.
- (a) C. M. Zaleski, E. C. Depperman, J. W. Kampf, M. L. Kirk and V. L. Pecoraro, Angew. Chem., Int. Ed., 2004, 43, 3912 CrossRef CAS PubMed; (b) J. Jankolovits, C. M. Andolina, J. W. Kampf, K. N. Raymond and V. L. Pecoraro, Angew. Chem., Int. Ed., 2011, 50, 9660 CrossRef CAS PubMed; (c) T. T. Boron, J. W. Kampf and V. L. Pecorao, Inorg. Chem., 2010, 49, 9104 CrossRef CAS PubMed; (d) C. M. Zaleski, J. W. Kampf, T. Mallah, M. L. Kirk and V. L. Pecoraro, Inorg. Chem., 2007, 46, 1954 CrossRef CAS PubMed; (e) A. D. Cutland, R. G. Malkani, J. W. Kampf and V. L. Pecoraro, Angew. Chem., Int. Ed., 2000, 39, 2689 CrossRef CAS; (f) A. D. Cutland, J. A. Halfen, J. W. Kampf and V. L. Pecoraro, J. Am. Chem. Soc., 2001, 123, 6211 CrossRef CAS; (g) C. S. Lim, J. W. Kampf and V. L. Pecoraro, Inorg. Chem., 2009, 48, 5224 CrossRef CAS PubMed; (h) A. D. Cutland-Van Noord, J. W. Kampf and V. L. Pecoraro, Angew. Chem., Int. Ed., 2002, 41, 4668 CrossRef PubMed; (i) C. M. Zaleski, A. D. Cutland-Van Noord, J. W. Kampf and V. L. Pecoraro, Cryst. Growth Des., 2007, 7, 1098 CrossRef CAS; (j) A. J. Stemmler, J. W. Kampf, M. L. Kirk, B. H. Atasi and V. L. Pecoraro, Inorg. Chem., 1999, 38, 2807 CrossRef CAS PubMed; (k) M. Tegoni, M. Furlotti, M. Tropiano, C. S. Lim and V. L. Pecoraro, Inorg. Chem., 2010, 49, 5190 CrossRef CAS PubMed; (l) C. S. Lim, J. Jankolovits, P. Zhao, J. W. Kampf and V. L. Pecoraro, Inorg. Chem., 2011, 50, 4832 CrossRef CAS PubMed; (m) C. M. Zaleski, C. S. Lim, A. D. C. V. Noord, J. W. Kampf and V. L. Pecoraro, Inorg. Chem., 2011, 50, 7707 CrossRef CAS PubMed; (n) J. T. Grant, J. Jankolovits and V. L. Pecoraro, Inorg. Chem., 2012, 51, 8034 CrossRef CAS PubMed; (o) J. Jankolovits, C. S. Lim, G. Mezei, J. W. Kampf and V. L. Pecoraro, Inorg. Chem., 2012, 51, 4527 CrossRef CAS PubMed; (p) C. S. Lim, M. Tegoni, T. Jakusch, J. W. Kampf and V. L. Pecoraro, Inorg. Chem., 2012, 51, 11533 CrossRef CAS PubMed; (q) J. Jankolovits, J. W. Kampf and V. L. Pecoraro, Inorg. Chem., 2013, 52, 5063 CrossRef CAS PubMed; (r) J. Jankolovits, J. W. Kampf and V. L. Pecoraro, Polyhedron, 2013, 52, 491 CrossRef CAS PubMed; (s) J. Jankolovits, A. D. C. Van-Noord, J. W. Kampf and V. L. Pecoraro, Dalton Trans., 2013, 42, 9803 RSC; (t) B. Ruggiero, M. Vivarelli, A. Gianviti, E. Benetti, L. Peruzzi, G. Barbano, F. Corona, G. Ventura, C. Pecoraro, L. Murer, G. M. Ghiggeri, M. Pennesi, A. Edefonti, R. Coppo and F. Emma, Nephrol., Dial., Transplant., 2013, 28, 1487 CrossRef CAS PubMed.
- M. Gajewska, K. V. Luzyanin, M. F. C. Guedes da Silva, Q. S. Li, J. R. Cui and A. J. L. Pombeiro, Eur. J. Inorg. Chem., 2009, 3765 CrossRef CAS.
- (a) D. Gaynor, Z. A. Starikova, W. Haase and K. B. Nolan, J. Chem. Soc., Dalton Trans., 2001, 1578 RSC; (b) A. B. M. Alagha, D. Gaynor, H. Muller-Bunz, K. B. Nolan and L. Parthasarathi, Acta Crystallogr., Sect. E: Struct. Rep. Online, 2010, 66, m853 CAS.
- (a) X. M. Shang, J. Z. Wu and Q. S. Li, Eur. J. Inorg. Chem., 2006, 4143 CrossRef CAS; (b) C. Mulcahy, F. M. Dolgushin, K. A. Krot, D. Griffith and C. J. Marmion, Dalton Trans., 2005, 1993 RSC.
- R. E. P. Winpenny, in Comprehensive Coordination Chemistry II, ed. J. A. McCleverty and T. J. Meyer, Elsevier, Amsterdam, 2004, vol. 7, pp. 125–175 Search PubMed.
- (a) Z. Chen, D. Luo, M. Kang and Z. Lin, Inorg. Chem., 2011, 50, 4674 CrossRef CAS PubMed; (b) S. Q. Bai, E. Q. Gao, Z. He, C. J. Fang and C. H. Yan, CrystEngComm, 2004, 6, 606 RSC; (c) J. A. Gould, J. T. A. Jones, J. Bacsa, Y. Z. Khimyak and M. J. Rosseinsky, Chem. Commun., 2010, 2793 RSC.
- (a) S. L. Zheng and X. M. Chen, Aust. J. Chem., 2004, 57, 703 CrossRef CAS; (b) X. Li, R. Cao, W. Bi, D. Yuan and D. Sun, Eur. J. Inorg. Chem., 2005, 3156 CrossRef CAS; (c) Y. Bai, J. L. Wang, D. B. Dang, M. M. Li and J. Y. Niu, CrystEngComm, 2012, 14, 1575 RSC.
- G. Yu, S. Yin, Y. Liu, Z. Shuai and D. Zhu, J. Am. Chem. Soc., 2003, 125, 14816 CrossRef CAS PubMed.
- (a) C. F. Lee, K. F. Chin, S. M. Peng and C. M. Che, J. Chem. Soc., Dalton Trans., 1993, 467 RSC; (b) Y. Ma, H. Y. Chao, Y. Wu, S. T. Lee, W. Y. Yu and C. M. Che, Chem. Commun., 1998, 22, 2491 RSC; (c) Y. Ma, T. Lai and Y. Wu, Adv. Mater., 2000, 12, 433 CrossRef CAS.
- (a) A. Erxleben, Coord. Chem. Rev., 2003, 246, 203 CrossRef CAS; (b) P. Jiang and Z. J. Guo, Coord. Chem. Rev., 2004, 248, 205 CrossRef CAS PubMed; (c) S. N. Wang, Coord. Chem. Rev., 2001, 215, 79 CrossRef CAS.
- (a) D. A. Brown, W. Errington, N. J. Fitzpatrick, W. K. Glass, T. J. Kemp, H. Nimir and Á. T. Ryan, Chem. Commun., 2002, 1210 RSC; (b) D. A. Brown, N. J. Fitzpatrick, H. Müller-Bunz and Á. T. Ryan, Inorg. Chem., 2006, 45, 4497 CrossRef CAS PubMed; (c) T. Tekeste and H. Vahrenkamp, Inorg. Chim. Acta, 2007, 360, 1523 CrossRef CAS PubMed.
- (a) X. Meng, Y. Song, H. Hou, H. Han, B. Xiao, Y. Fan and Y. Zhu, Inorg. Chem., 2004, 43, 3528 CrossRef CAS PubMed; (b) S. Biswas, M. Maes, A. Dhakshinamoorthy, M. Feyand, D. E. De Vos, H. Garcia and N. Stock, J. Mater. Chem., 2012, 22, 10200 RSC.
- M. Alexiu, C. Dendrinou-Samara, C. P. Raptopoulou, A. Terzis and D. P. Kessissoglou, Inorg. Chem., 2002, 41, 4732 CrossRef PubMed.
- G. M. Sheldrick, SHELXS–97, Program for Crystal Structure Solution, University of Göttingen, Germany, 1997 Search PubMed.
- G. M. Sheldrick, SHELXL–97, Program for the Refinement of Crystal Structures from Diffraction Data, University of Göttingen, Germany, 1997 Search PubMed.
- V. A. Blatov, Nanocluster analysis of intermetallic structures with the program package TOPOS, Struct. Chem., 2012, 23, 955 CrossRef CAS PubMed.
- (a) J. H. Yang, W. Li, S. L. Zheng, Z. L. Huang and X. M. Chen, Aust. J. Chem., 2003, 56, 1175 CrossRef CAS; (b) S. L. Zheng, J. P. Zhang, X. M. Chen, Z. L. Huang, Z. Y. Lin and W. T. Wong, Chem.–Eur. J., 2003, 9, 3888 CrossRef CAS PubMed; (c) S. L. Zheng, J. H. Yang, X. L. Yu, X. M. Chen and W. T. Wong, Inorg. Chem., 2004, 43, 830 CrossRef CAS PubMed; (d) L. L. Wen, D. B. Dang, C. Y. Duan, Y. Z. Li, Z. F. Tian and Q. J. Meng, Inorg. Chem., 2005, 44, 7161 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Tables of selected bond lengths and angles, and additional figures of these compounds. CCDC 882293, 948225, 948226, and 948228 for complexes 1 to 4, respectively. For crystallographic data in CIF or other electronic format see DOI: 10.1039/c3ra44489a |
| This journal is © The Royal Society of Chemistry 2014 |