Catalytic activity and selectivity of reusable α-MoO3 nanobelts toward oxidation of olefins and sulfides using economical peroxides†
Abstract
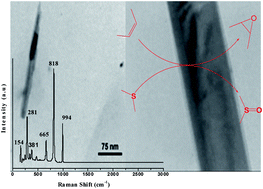
The novel catalytic activity of α-MoO3 nanobelts prepared by a new and safe sol–gel method for the epoxidation of olefins and oxidation of sulfides to sulfoxides using H2O2 in ethanol as a safe solvent has been exploited. The reactions also proceeded efficiently in the presence of tert-butyl hydroperoxide (TBHP). Good/high yields and excellent selectivity resulted. The ammonia TPD profile demonstrated strong acidic sites in synthesized α-MoO3 nanobelts, which generated different catalytic activity than the bulk material. The separation and reuse of this heterogeneous nanocatalyst was simple, effective and economical in the presented oxidation methods.

 Please wait while we load your content...
Please wait while we load your content...