Investigation of antimicrobial PEG-poly(amino acid)s†
Abstract
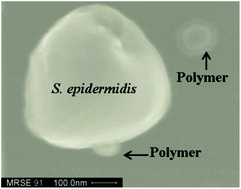
There has been significant interest in the development of antimicrobial cationic polymers due to their low manufacture cost and ease of synthesis compared to small antimicrobial peptides (AMPs). These polymers are designed to mimic amphiphilic structures of AMPs which can disrupt negatively charged bacterial membranes, and can therefore lead to potential antibiotic agents to fight emerging drug resistance. However, the reports of biodegradable antimicrobial polymer nanoparticles are rare. Herein we report the development of antimicrobial PEG-poly(amino acid)s. Some of these multi-block PEG-poly(amino acid)s form defined nanoparticles in solution, and display potent and broad-spectrum antimicrobial activity. Fluorescence and SEM studies show that these polymers are likely to kill bacteria by disrupting bacterial membranes. As these polymers are biodegradable and easy to scale up, they may provide an attractive approach for the development of antibiotic agents.

 Please wait while we load your content...
Please wait while we load your content...