Fabrication of porous carbon spheres for high-performance electrochemical capacitors†
Jie Wang,
Laifa Shen,
Bing Ding,
Ping Nie,
Haifu Deng,
Hui Dou and
Xiaogang Zhang*
College of Materials Science and Engineering and Key Laboratory for Intelligent Nano Materials and Devices of the Ministry of Education, Nanjing 210016, P.R. China. E-mail: azhangxg@163.com; Fax: +86 025 52112626; Tel: +86 025 52112902
First published on 9th January 2014
Abstract
Porous carbon spheres (PCS) with meso/microporous structure are designed and fabricated through a facile hydrothermal method by employing glucose as a carbon precursor and sodium molybdate (Na2MoO4) as a porogen, structure-direct agent and catalyst. With the assistance of Na2MoO4, the porous structure of the carbon sphere is significantly enhanced. In addition, the meso/microporous structure can be modulated by adjusting the proportion of the reactants. In optimal conditions, the PCS exhibit a high specific surface area (SSA, 757.3 m2 g−1) and pore volume (0.24 cm3 g−1). When evaluated as an electrode for electrochemical capacitors, the PCS exhibits a high specific capacitance of 260 F g−1 with remarkable high-rate performance and long-term cycling stability. The excellent electrochemical performances are exclusively attributed to the micro/mesoporous structure, which maximize the ion accumulation on the electrode surface and facilitate fast ion transportation. The well-defined porous nanostructure plus easy strategy make current study provide new opportunities for hydrothermal carbonization of biomass as electrode materials for energy storage.
Introduction
Electrochemical capacitors (ECs), also well known as supercapacitors, are considered as promising energy-storage devices in customer electronics, communication, and transportation.1–3 ECs could be broadly divided into faradaic pseudocapacitors and double electric layer capacitors (EDLCs). The materials for pseudocapacitors are mainly transition metal oxides and conducted polymers, the contribution of which comes from the reversible oxygen reduction reaction occurring on the surface of electrodes.4,5 The double layer utilized by EDLCs are formed at electrolyte/electrode interfaces where electric charges are accumulated on the electrode surfaces and ions of opposite charge are arranged in the electrolyte side.6 Such energy-storage mechanism permits EDLCs with several desirable advantages such as high power density and excellent cycling life span in comparison with modern secondary batteries.7 EDLCs electrode materials should thus have a large specific surface area (SSA) and match with appropriate pore structure for charge accumulation, electrolyte wetting and rapid ionic transportation.8,9Carbon materials, such as activated carbon (AC), graphene, carbon nanotubes (CNTs), and mesoporous carbon, are conventionally used as electrode materials for EDLCs because of their advantages such as large SSA, high electric conductivity, porous structure, excellent cycling stability and low cost.2,10,11 The most widely adopted electrode material is AC. However, the performance would be raised if the limited surface area accessible to electrolyte ions in AC could be improved.12 Mesoporous carbon, especially ordered mesoporous carbon (OMC), with a narrow distribution and a uniform pore connection have much better electrochemical performance than conventional AC at high current densities. Because these mesopore channels and interconnections provide a more favorable path for penetration and transportation of ions.13 However, the lack of micropores hinders the further improvement of energy density of such material.14,15 Chemical modified graphene has also been widely studied as energy-storage material for its multiple functional groups and two-dimensional morphology.16,17
To advance the performance of porous carbon-based ECs, porous structures are expected to be an optimized choice for EDLCs electrode. The mesopores could serve as channels for fast electrolyte ions supply and electron transfer, thus reduce the resistance caused by the concentration polarization effect.18 The micropores in such structure will supply high surface area for the adsorption of electrons and thus strengthen the EDLCs.19 In recent years, the combinations of hard templates, (such as PS/PMMA/SiO2 spheres, AAO membranes and MnO2 nanotubes), with structure-directing agents F127 or P123 are extensively employed to prepare different morphological carbons with hierarchical porosity while organic resols are taken as carbon precursors.13,20–23 However, the above mentioned methods involve multi-step complicated preparation route of synthesis of hard templates, self-assembly, removing of the templates, which severely limit their wide application. Therefore, fabrication of porous nanostructure through a facile, low-cost, and environmentally friendly route is still a challenge. Among various techniques, the hydrothermal carbonization process of biomass exhibits lots of advantages for the synthesis of novel carbon-based materials.24–27 An increasing number of researches focus on hydrothermal carbonization of the isolated carbohydrates and crude plant. Markus Antonietti et al. have reported the production of porous carbon for supercapacitor electrode through hydrothermal treatment of glucosamine at 180 °C.28,29 Another example is the use of grass for the synthesis of carbon materials.30 The adoption of easy-obtained biomass as precursor avoids complicated preparation and cumbersome treatment. But the shape need to be modified and the porous structure should be modulated for rapid ion diffusion and charge accumulation. Then it will be much more suitable for practical application of the EDLCs.
In this paper, we successfully prepared highly dispersed carbon spheres with porous nanostructure by employing glucose as carbon source and Na2MoO4 as porogen. Except the abundant porous structure and the well-kept morphology, the pore structure could be modulated by adjusting the proportion of reactants. The porous carbon spheres (PCS) has been evaluated as EDLCs electrode in aqueous KOH solution and their remarkable capacitive performance has been demonstrated in this work.
Experimental
Preparation of PCS
Highly dispersed carbon spheres with porous structure were synthesized as described followed. In a typical synthesis, X mg (X = 70, 120, 170) of sodium molybdate (Na2MoO4) (Aladdin) and 1.08 g of D-glucose (C6H12O6, Aladdin) was dissolved in 10.0 mL 0.5 M HCl solution and stirred for 1 h at room temperature. Afterwards, the solution was transferred to a Teflon-lined stainless steel autoclave and hydrothermally treated at 180 °C for 6 h. After cooling down naturally, the dark product precipitate was washed thoroughly with deionized water and ethanol and then dried at 60 °C. The as-synthesized sample was denoted as Mo/C-1. The Mo/C-1 sample was then loaded on a porcelain boat, which was placed in the centre of a quartz tube heated by a tube furnace. The pyrolysis of Mo/C-1 was conducted in a nitrogen atmosphere at 800 °C for 4 h at a heating rate of 5 °C min−1; the resultant sample from this carbonization process was named as Mo/C-2. The Mo/C-2 was further heat-treated (or oxidized) in air at 300 °C for 3 h at a heating rate of 1 °C min−1, and the resulted sample was denoted as Mo/C-3. Thereafter, the Mo/C-3 was immersed in ammonia and stirred to remove the oxides to harvest the final product PCS. The resultant samples obtained from different amounts of Na2MoO4 were named as PCS-X.Material characterization
Field emission scanning electron microscopy (FESEM) was carried out with a JEOL JSM-6380LV FESEM. Powder X-ray diffractions (XRD) of the samples were studied by Bruker D8 Advance X-ray diffractometer using Cu Kα radiation. The X-ray photoelectron spectroscopy (XPS) analysis was performed on a Perkin-Elmer PHI 550 spectrometer with Al Kα (1486.6 eV) as the X-ray source. Brunauer–Emmett–Teller surface area measurements were carried out with a volumetric sorption analyser (NOVA 2000, Quantachrome) using physical adsorption–desorption of nitrogen gas at the liquid-nitrogen temperature. Pore size distributions (PSD) were determined from the sorption isotherms using a non-local density functional theory (NLDFT) assuming pores are slit shaped. Micropore surface area and volume were estimated by Dubinin–Radushkevitvh (DR) equation. Thermal gravimetric analysis (TGA) was conducted on a TG-DSC instrument (NETZSCH STA 409 PC) under air at a heating rate of 10 °C min−1 from 30 to 800 °C.Electrochemical measurements
All the electrochemical measurements were carried out in a conventional three-electrode system with 6 M KOH aqueous solution as the electrolyte. Platinum foil and a saturated calomel electrode (SCE) were used as counter and reference electrodes, respectively. The working electrodes were prepared by mixing active material (5 mg), acetylene black and polytetrafluorene-ethylene (PTFE) binder with a weight ratio of 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15
15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5. After coating the above slurries on foamed Ni grids (1 cm × 1 cm), the electrodes were dried at 60 °C for several hours before pressing under a pressure of 15 MPa. A CHI660C electrochemical working station instrument (Shanghai Chenhua, China) was employed for cyclic voltammetry (CV), galvanostatic charge–discharge and electrochemical impedance spectroscopy (EIS). The Nyquist impedance spectrums were recorded in the frequency range of 0.01 Hz to 100 kHz at the open circuit potential with 5 mV amplitude.
5. After coating the above slurries on foamed Ni grids (1 cm × 1 cm), the electrodes were dried at 60 °C for several hours before pressing under a pressure of 15 MPa. A CHI660C electrochemical working station instrument (Shanghai Chenhua, China) was employed for cyclic voltammetry (CV), galvanostatic charge–discharge and electrochemical impedance spectroscopy (EIS). The Nyquist impedance spectrums were recorded in the frequency range of 0.01 Hz to 100 kHz at the open circuit potential with 5 mV amplitude.
Results and discussion
As illustrated in Scheme 1, the general preparation process of PCS includes hydrothermal reaction of glucose and Na2MoO4, calcinations and removing the porogen. During the hydrothermal carbonization, the initial reactants are transformed into Mo/C-1 composite with nanoparticles dispersing and embedding in the carbon spheres. The reaction time of 6 h was chosen according to the reported paper.31 At this condition, the intermediate products highly dispersed in the carbon spheres are in the size of 2–6 nm, which is appropriate to be porogen for mesopores. The spheres are turned into Mo/C-2 and Mo/C-3 after pyrolysis in the nitrogen and air, respectively. After removing the templates embedded in carbon spheres, PCS are obtained. The mass yield is about 36 wt%, which is calculated by the ratio of the produced carbon and glucose.The results of XRD analysis of Mo/C are presented in Fig. 2. For Mo/C-1, the diffraction peaks at 26°, 37°, 54°, 60° and 66° prove the formation of molybdenum dioxide (MoO2) while the peak at 41° is assigned to the appearance of molybdenum carbide (Mo2C). These components should be productions of Na2MoO4, restored by the glucose during the hydrothermal reaction. According to the analysis of Mo/C-2, the peaks are significantly intensified after carbonization at 800 °C in nitrogen, indicating of the increase of crystallinity degree. Possibly it is caused by the reduction of carbon in the inert gas. The disappeared MoO2 and Mo2C are oxidized into molybdenum trioxide (MoO3) after calcinations at 300 °C in air. In addition, we found that after oxidation in air, the FWHM (full width at half maximum) of MoO3 is broadened. According to the Scherrer formula,32 we could calculate that the sizes of MoO3 embedded in the carbon sphere matrix range from 2 to 4 nm. Thus the diameter of the pores which are created by these oxides could be deduced. It will be further confirmed by the analysis of the N2 adsorption. Based on XRD results, MoO3 has been completely resolved after immersing in ammonia and the amorphous carbon, the PCS, is produced. It is further verified by TGA curve that the templates have been removed (Fig. S1†).
As observed from Fig. 2a, the carbon spheres are enwrapped by the nanoparticles which are dispersed uniformly on the spheres. Except the particles, the surface of carbon spheres is demonstrated to be smooth in Fig. 1b and c. The particles were likely formed during the cooling of hydrothermal process due to increase in degree of supersaturation when temperature decreased.33,34 Actually the interplay between MoO2 formation and D-glucose oxidation plays an important role in producing spherical biphasic nanocomposites, noting that molybdenum precursor Na2MoO4 is an oxidant in this redox process under hydrothermal conditions. In addition, acid has been taken here to control the precipitation kinetics of both metal oxide and porous carbonaceous matrixes under hydrothermal conditions, which is crucial to form uniform carbon encapsulated spheres.31 In Fig. 1d and e, the nanoparticles have disappeared after immersing in ammonia and the surface of carbon spheres turns to be rough. The magnified picture of carbon spheres is shown in Fig. 1f and the pores can be clearly observed. It can be deduced that the remove of templates exactly produce the porous structures, which may contribute to the transfer of ions and reduction of the overall resistance. In comparison, we prepared carbon spheres through hydrothermal reaction of glucose. The products are smooth spheres without any obvious pores on the surface and the period of hydrothermal reaction is ten hours longer than the reaction with Na2MoO4.
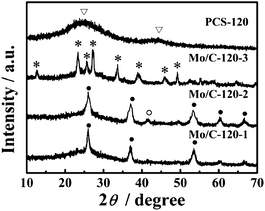 | ||
| Fig. 1 XRD patterns of Mo/C-1, Mo/C-2, Mo/C-3 and PCS. Peaks marked with symbols •, ○, * and ▽ correspond to MoO2, Mo2C, MoO3 and hexagonal graphitic carbon, respectively. | ||
To insight into the effects of porogen on the porosity characteristics of PCS, N2 adsorption–desorption isotherms were conducted. Fig. 3 compares the N2 adsorption–desorption isotherms and the corresponding PSD of PCS samples and glucose-C. As depicted in Fig. 3a, the N2 adsorption–desorption isotherms of PCS-70, 120 and 170 exhibit the characteristics of type IV with a sharp increase at low pressure (P/P0 ≤ 0.01) and clear hysteresis loops at high relative pressure (P/P0 > 0.01), indicating the coexistence of micropores and mesopores.35 The N2 adsorption–desorption isotherms of glucose-C exhibits sharp increase at low pressure but with no hysteresis loops.
 | ||
| Fig. 3 (a) N2 adsorption–desorption isotherms, (b) the pore size distribution of PCS samples and glucose-C. | ||
The SSA and pore sizes of PCS materials and glucose-C are summarized in Table 1. The PCS-120 exhibits a high SSA of 757.3 m2 g−1, which is nearly three times higher than that of glucose-C (219.9 m2 g−1). In particular, the contribution of mesopores in the PSC significantly increases. For PSC-70, 120 and 170, the mesopores surface area is about 25, 32 and 31% of total SSA. While for glucose-C the ratio is only about 13%. The corresponding PSD curves (Fig. 3b) provide detailed information about the distribution of different size pores. For PCS-70 and 120, the pore sizes of mesopores are mainly centered at 2.1 nm. In contrast, the glucose-C shows no obvious mesopores. The abundant mesopores of PCS-120 are mainly resulted from the remove of the porogen and the mesopore size is consistent to the XRD results quite well. Analysed by DR equation, micropore sizes of PCS-120 and glucose-C both center at about 0.5 nm. The inner pore structure of PCS is illustrated in Fig. 3d. The porogen increase the SSA and build porous nanostructure with co-existence of mesopores and micropores.
| Samples | SSA [m2 g−1] | Meso-BET [m2 g−1] | Micro-pore size [nm] | Meso-pore size [nm] | Pore volume [cm3 g−1] | Specific capacitance [F g−1] |
|---|---|---|---|---|---|---|
| Glucose-C | 219.9 | 28.6 (13%) | 0.5 | 1.5 | 0.12 | 151 |
| PCS-70 | 412.1 | 102.2 (25%) | 0.5 | 2.1 | 0.18 | 125 |
| PCS-120 | 757.3 | 242.3 (32%) | 0.5 | 2.1 | 0.24 | 230 |
| PCS-170 | 524.7 | 162.7 (31%) | 0.5 | 2.4 | 0.22 | 187 |
Obviously, the porogen increase the porosity of glucose derived carbon spheres. When the final products (MoO3 obtained from Na2MoO4) are removed, the mesopores are created. In addition, much more gases released during the reaction between Na2MoO4 and glucose generate more micropores and further improves the SSA. The produced porous structure can reduce the internal resistance and then meet the requirements of rapid migration of the electrolyte ions. The surface areas could thus be fully utilized to form the electric double layer. With the increasing amount of Na2MoO4, more pores are produced and higher SSA are created. However, when the quantity of Na2MoO4 to react with glucose reaches saturation, the excess reactants dispersed randomly may produce more conjoint oxides. These oxides would cause the collapse of mesopores and thus lead to decreasing SSA. That is why the surface area of the PCS-170 is lower than that of the PCS-120.
The composition of the PCS-120 sample is further determined by XPS technique. The contents of carbon and oxygen are 87.56%, and 12.44%, respectively. It is demonstrated all the inorganic species have been removed (Fig. S2†). As shown in Fig. 4, the C1s spectrum ranging from 280.0 to 295.0 eV can be approximately fitted into three peaks, centering at ca. 284.2, 285.1, and 288.7 eV. The peak at ca. 284.2 eV corresponds to sp2 C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond of graphitic carbon, while the one at ca. 285.1 eV is attributed to sp3 C–C bond. Regarding the peak at ca. 288.7 eV, it can be ascribed to –C
C bond of graphitic carbon, while the one at ca. 285.1 eV is attributed to sp3 C–C bond. Regarding the peak at ca. 288.7 eV, it can be ascribed to –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O/–COOH bonds.36 The O1s spectrum in Fig. 4 can also be fitted into three peaks locating at ca. 531.3, 532.5, and 533.4 eV. The peak at ca. 531.3 eV can be indexed as C–O–C/C–OH bond;37 the peaks at ca. 532.5 eV and ca. 533.4 eV are assigned to –C
O/–COOH bonds.36 The O1s spectrum in Fig. 4 can also be fitted into three peaks locating at ca. 531.3, 532.5, and 533.4 eV. The peak at ca. 531.3 eV can be indexed as C–O–C/C–OH bond;37 the peaks at ca. 532.5 eV and ca. 533.4 eV are assigned to –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and –C–O bonds, respectively.38
O and –C–O bonds, respectively.38
To investigate the electrochemical performances of the as-prepared PCS materials for ECs electrodes, the samples are characterized by CV and galvanostatic charge–discharge measurements. Fig. 5a shows the CV curves of the PCS-120 at various scan rates ranging from 5 to 100 mV s−1. All CV curves are close to rectangular shapes in a potential range of −1–0 V, an indication of mainly double layer capacitive behavior and fast diffusion of electrolyte ions into/out the electrode materials. Fig. 5b compares the CV curves of glucose-C and PCS samples at a scan rate of 5 mV s−1. The glucose-C exhibits an almost ideally rectangular shape, showing almost mirror images with respect to the zero-current line, which is characteristic of an EDLC. The CV curves of PCS samples deviate from perfect rectangular shape with a few humps. This observation is caused by the pseudocapacitive behaviour of the oxygen-containing functional groups on the surface, which to some extent accords with the XPS values shown in Fig. 4.39,40 Furthermore, PCS-70 and glucose-C electrodes exhibits lower current corresponding, which could be attributed to the lower SSA and limited ion incorporation into the active material. As depicted in the charge–discharge curves (Fig. 5c), the discharging time of the PCS-120 is significantly longer compared with that of other materials. The specific capacitances of the electrodes are calculated by equation:
The relationships of capacitance and current density are described in Fig. 5d. There is a slight decrease for PCS-120 when the current density transfers from 0.5 to 1 A g−1, but the capacitance maintains well even under high current density. In the range of 1 to 10 A g−1, the capacitance of PCS-120 changes from 230 to 171 F g−1 while the capacitance of glucose-C electrode decreases from 151 to 86 F g−1. The enhanced rate performances of PCS-120 indicate faster charge transfer and ion transport during the charge–discharge process. For PCS-120, meso/microporous structure provides a shorter ion diffusion length and a faster mass transport channel. The optimized porous structure is critical to the superior capacitive performances of PCS-120, which permits highly conductive pathway for electrons and fast ion transport channel. To grasp further insights into to the effects of the pore structure on the electrochemical performances, EIS of the sample with different pore characterization was measured.
The Nyquist impedance spectrums of the PCS-70, PCS-120, PCS-170 and glucose-C electrodes are presented in Fig. 5a and b. It can be seen that the impedance plots for all of the fully charged states were composed of a depressed semicircle in the high-frequency region and a sloping straight line in the low-frequency region. The semicircle in the high-frequency region corresponds to the charge-transfer process, and the straight line in the low-frequency region corresponds to a semi infinite Warburg diffusion process.45,46 Obviously, the glucose-C electrode exhibit larger resistance than that of PCS electrodes. It is because a resistive component mainly due to a hindered mobility of ions in micropores and/or a low electrical conductivity of the electrode, is involved during the charging process. The impact of resistive component becomes smaller as the SSA increases. The PCS-120 sample shows the lowest resistance and the highest capacitance, compared with the other samples, which is certainly relative with its highest values of SSA and pore volume, as displayed in Table 1.
Cycling performance is of great importance for ECs. The long-term stability of the PCS-120 electrode was examined by galvanostatic charge–discharge cycling at a current density of 1 A g−1, and the results are presented in Fig. 6. After 2000 cycles, the specific capacitance decrease from 260.3 to 246.7 F g−1, remaining about 95% of the initial capacitance. The PCS-120 electrode is proved to display excellent cycling stability (Fig. 7).
 | ||
| Fig. 6 (a) EIS spectra of PCS-70, PCS-120 and PCS-170 (the inset shows the spectra at high-frequency region), (b) EIS spectra of glucose-C. | ||
 | ||
| Fig. 7 Dependences of the specific capacitance on the charge–discharge cycle numbers of the PCS-120 at a current density of 1 A g−1. | ||
Conclusions
In summary, a series of PCS with porous structure were successfully prepared with glucose as carbon precursor and Na2MoO4 as porogen. The pore-structure analysis showed that the SSA and pore volume could be tuned by adjusting ratio of initial reactants. The PCS exhibited a specific surface area of 757.3 m2 g−1 and a porous structure under optimal condition, with a primary mesopore size of 2.1 nm and micropore sizes centered at 0.5 nm. The electrochemical measurements demonstrated that the porous structure of PCS and abundant functional groups endowed the carbon spheres with good capacitive performance, that is, a high electrochemical capacitance of 260 F g−1, excellent high rate performance and good long-term cycling stability. Such an excellent electrocapacitive performance indicated that the PCS is a kind of promising electrode material for the EDLCs.Acknowledgements
This work was supported by the National Basic Research Program of China (973 Program) (no. 2014CB239701), National Natural Science Foundation of China (no. 21173120, 21103091,51372116), Natural Science Foundation of Jiangsu Province (BK2011030).Notes and references
- J. R. Miller and P. Simon, Science, 2008, 321, 651–652 CrossRef CAS PubMed.
- L. L. Zhang and X. S. Zhao, Chem. Soc. Rev., 2009, 38, 2520–2531 RSC.
- C. Z. Yuan, B. Gao, L. F. Shen, S. D. Yang, L. Hao, X. J. Lu, F. Zhang, L. J. Zhang and X. G. Zhang, Nanoscale, 2011, 3, 529–545 RSC.
- M. Liu, J. Chang, J. Sun and L. Gao, RSC Adv., 2013, 3, 8003–8008 RSC.
- L. L. Zhang, S. Zhao, X. N. Tian and X. S. Zhao, Langmuir, 2010, 26, 17624–17628 CrossRef CAS PubMed.
- S. Yoon, J. Lee, T. Hyeon and S. M. Oh, J. Electrochem. Soc., 2000, 147, 2507–2512 CrossRef CAS PubMed.
- J. Zhang and X. S. Zhao, ChemSusChem, 2012, 5, 818–841 CrossRef CAS PubMed.
- A. G. Pandolfo and A. F. Hollenkamp, J. Power Sources, 2006, 157, 11–27 CrossRef CAS PubMed.
- C. Largeot, C. Portet, J. Chmiola, P. L. Taberna, Y. Gogotsi and P. Simon, J. Am. Chem. Soc., 2008, 130, 2730–2731 CrossRef CAS PubMed.
- S. L. Candelaria, Y. Shao, W. Zhou, X. Li, J. Xiao, J. G. Zhang, Y. Wang, J. Liu, J. Li and G. Cao, Nano Energy, 2012, 1, 195–220 CrossRef CAS PubMed.
- E. Frackowiak, Phys. Chem. Chem. Phys., 2007, 9, 1774–1785 RSC.
- L. L. Zhang and X. S. Zhao, Chem. Soc. Rev., 2009, 38, 2520–2531 RSC.
- H. J. Liu, W. J. Cui, L. H. Jin, C. X. Wang and Y. Y. Xia, J. Mater. Chem., 2009, 19, 3661–3667 RSC.
- W. Xing, C. C. Huang, S. P. Zhuo, X. Yuan, G. Q. Wang, D. Hulicova-Jurcakova, Z. F. Yan and G. Q. Lu, Carbon, 2009, 47, 1715–1722 CrossRef CAS PubMed.
- Y. Luan, Y. W. Xue and Z. G. Shi, Mater. Lett., 2012, 88, 30–32 CrossRef CAS PubMed.
- D. Wu, F. Zhang, H. Liang and X. Feng, Chem. Soc. Rev., 2012, 41, 6160–6177 RSC.
- D. Wu, F. Zhang, P. Liu and X. Feng, Chem. – Eur. J., 2011, 17, 10804–10812 CrossRef CAS PubMed.
- H. Zhang, X. Yu and P. V. Braun, Nat. Nanotechnol., 2011, 6, 277–281 CrossRef CAS PubMed.
- Z. Lei, N. Christov, L. L. Zhang and X. S. Zhao, J. Mater. Chem., 2011, 21, 2274–2281 RSC.
- C. H. Huang, Q. Zhang, T. C. Chou, C. M. Chen, D. S. Su and R. A. Doong, ChemSusChem, 2012, 5, 563–571 CrossRef CAS PubMed.
- J. Schuster, G. He, B. Mandlmeier, T. Yim, K. T. Lee, T. Bein and L. F. Nazar, Angew. Chem., Int. Ed., 2012, 51, 3591–3595 CrossRef CAS PubMed.
- G. Zhao, J. He, C. Zhang, J. Zhou, X. Chen and T. Wang, J. Phys. Chem. C, 2008, 112, 1028–1033 CAS.
- H. Liu, L. H. Jin, P. He, C. Wang and Y. Xia, Chem. Commun., 2009, 6813–6815 RSC.
- B. Hu, K. Wang, L. Wu, S. H. Yu, M. Antonietti and M. M. Titirici, Adv. Mater., 2010, 22, 813–828 CrossRef CAS PubMed.
- M. M. Titirici, M. Antonietti and N. Baccile, Green Chem., 2008, 10, 1204–1212 RSC.
- P. J. Hall, M. Mirzaeian, S. I. Fletcher, F. B. Sillars, A. J. R. Rennie, G. O. Shitta-Bey, G. Wilson, A. Cruden and R. Carter, Energy Environ. Sci., 2010, 3, 1238–1251 CAS.
- M. M. Titirici, A. Thomas, S. H. Yu, J. O. Müller and M. Antonietti, Chem. Mater., 2007, 19, 4205–4212 CrossRef CAS.
- L. Zhao, L. Z. Fan, M. Q. Zhou, H. Guan, S. Qiao, M. Antonietti and M. M. Titirici, Adv. Mater., 2010, 22, 5202–5206 CrossRef CAS PubMed.
- F. Rodríguez-Reinoso and M. Molina-Sabio, Carbon, 1992, 30, 1111–1118 CrossRef.
- S. Liu, J. Tian, L. Wang, Y. Zhang, X. Qin, Y. Luo, A. M. Asiri, A. O. Al-Youbi and X. Sun, Adv. Mater., 2012, 24, 2037–2041 CrossRef CAS PubMed.
- J. Dou and H. C. Zeng, J. Phys. Chem. C, 2012, 116, 7767–7775 CAS.
- J. Zhu, Z. Lu, S. T. Aruna, D. Aurbach and A. Gedanken, Chem. Mater., 2000, 12, 2557–2566 CrossRef CAS.
- X. W. Lou, J. S. Chen, P. Chen and L. A. Archer, Chem. Mater., 2009, 21, 2868–2874 CrossRef CAS.
- G. Yu, B. Sun, Y. Pei, S. Xie, S. Yan, M. Qiao, K. Fan, X. Zhang and B. Zong, J. Am. Chem. Soc., 2009, 132, 935–937 CrossRef PubMed.
- E. G. Calvo, N. Ferrera-Lorenzo, J. A. Menéndez and A. Arenillas, Microporous Mesoporous Mater., 2013, 168, 206–212 CrossRef CAS PubMed.
- T. I. T. Okpalugo, P. Papakonstantinou, H. Murphy, J. McLaughlin and N. M. D. Brown, Carbon, 2005, 43, 153–161 CrossRef CAS PubMed.
- C. Pevida, T. C. Drage and C. E. Snape, Carbon, 2008, 46, 1464–1474 CrossRef CAS PubMed.
- T. Horikawa, N. Sakao, T. Sekida, J. I. Hayashi, D. D. Do and M. Katoh, Carbon, 2012, 50, 1833–1842 CrossRef CAS PubMed.
- G. H. Moon, Y. Shin, D. Choi, B. W. Arey, G. J. Exarhos, C. Wang, W. Choi and J. Liu, Nanoscale, 2013, 5, 6291–6296 RSC.
- J. Sánchez-González, F. Stoeckli and T. A. Centeno, J. Electroanal. Chem., 2011, 657, 176–180 CrossRef PubMed.
- T. A. Centeno and F. Stoeckli, Electrochim. Acta, 2006, 52, 560–566 CrossRef CAS PubMed.
- J. A. Fernández, S. Tennison, O. Kozynchenko, F. Rubiera, F. Stoeckli and T. A. Centeno, Carbon, 2009, 47, 1598–1604 CrossRef PubMed.
- X. Y. Cheng, C. Chen, Z. J. Zhang and D. H. Xie, J. Mater. Chem. A, 2013, 1, 7379–7383 Search PubMed.
- J. B. Zhang, L. J. Jin, J. Cheng and H. Q. Hu, Carbon, 2013, 55, 221–232 CrossRef CAS PubMed.
- L. Wei, M. Sevilla, A. B. Fuertes, R. Mokaya and G. Yushin, Adv. Energy Mater., 2011, 1, 356–361 CrossRef CAS.
- D. Li, M. B. Muller, S. Gilje, R. B. Kaner and G. G. Wallace, Nat. Nanotechnol., 2008, 3, 101–105 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c3ra44305a |
| This journal is © The Royal Society of Chemistry 2014 |





