Surface confined successive growth of silver nanoplates on a solid substrate with tunable surface plasmon resonance†
Abstract
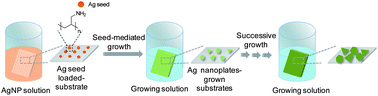
A strategy for direct growth of Ag nanoplates on a solid substrate was developed by using successive seed-mediated growth approach. The size and surface density of grown Ag nanoplates increased by repeated successive growth with tunable LSPR properties and the prepared Ag nanoplate grown substrates showed excellent performance as a SERS platform.

 Please wait while we load your content...
Please wait while we load your content...