DOI:
10.1039/C3RA44196B
(Paper)
RSC Adv., 2014,
4, 4609-4618
In(OTf)3-catalyzed chemoselective alkylation of tryptamines with 2-oxo-1-pyrrolidine derivatives†
Received
6th August 2013
, Accepted 17th October 2013
First published on 18th October 2013
Abstract
The chemoselective alkylation of tryptamine derivatives with 2-oxo-1-pyrrolidine compounds, catalyzed by In(OTf)3, was investigated. A series of 2-alkyl and N-alkyl tryptamine derivatives were synthesized under mild conditions with good total yields (up to 99%) and chemoselectivity (up to 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5).
5).
Introduction
The tryptamine skeleton widely exists in many natural products as well as in commercial drugs.1 Selected alkaloids bearing a tryptamine skeleton are shown in Scheme 1. Serotonin (1) is a neurotransmitter which influences the human nervous system and plays a part in central nervous system function.2 Melatonin (2), a neurohormone mainly secreted by the pineal gland, plays a pivotal role in regulating our body's circadian rhythms.3 Luzindole (3) is widely known as a reference MT2-selective melatonin receptor antagonist.4 Desformylflustrabromine (4) has been identified as the first selective allosteric modulator of nicotinic acetylcholine (nACh) receptors.5 Psychotrimine (5) and its analogous compounds often exhibit potent biological activity including anti-bacterial, anti-fungal, anti-viral and analgesic properties.6 Vinblastine (6), isolated from Catharanthus roseus at the end of the 1950s,7 is an efficient cancer chemotherapeutic agent in the therapy of particularly fast growing tumor types (leukemia, lymphoma).8
 |
| | Scheme 1 Representative alkaloids. | |
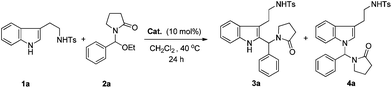
Recently, we reported iodine-catalyzed alkylation of indoles with 1-(ethoxy-aryl-methyl)pyrrolidin-2-one to form 3-alkylated indole derivatives under mild conditions.9 As a continuation of this project, we attempted to utilize the tryptamine derivative 1a with 1-(ethoxy-aryl-methyl)pyrrolidin-2-one 2a (Scheme 2).
 |
| | Scheme 2 Proposed attempt. | |
As expected, a 2-alkylized tryptamine derivative (3a) and an N-alkylized tryptamine derivative (4a) were obtained as the major and minor products, respectively.
In recent decades, numerous methods have been developed for the functionalization of tryptamine derivatives, such as the classical Fischer indolization, Larock's method,10 Friedel–Craft conjugate addition,11 and nitroethylation of indole derivatives followed by reduction.12 In addition, Nicolaou and coworkers have also covered a new synthetic approach from N-protected anilines to the functional tryptamines.13 It's still a challenge to functionalize the C-2 position of tryptamines directly; some methods referring to the synthesis of 2-benzyl tryptamines have reported limitations such as expensive catalysts, multiple steps, or harsh reaction conditions.14 Herein, we describe the chemoselective alkylation of tryptamines with 2-oxo-1-pyrrolidine derivatives, to give the desired C-2 alkylation of tryptamines bearing 2-oxo-1-pyrrolidine as the major products in good yields under mild conditions (Scheme 3).
 |
| | Scheme 3 Routes to 2-substituted tryptamine compounds. | |
Results and discussion
Our studies were initiated by the model reaction of N-tosyl tryptamine 1a with 2a in CH2Cl2 at 40 °C. As shown in Table 1, the model reaction proceeded well, especially in the presence of 10 mol% In(OTf)3 for 24 hours, which produced the 2-alkylized tryptamine derivative 3a in 63% LC-yield and the N-alkylized tryptamine derivative 4a in 5% LC-yield, respectively (Table 1, entry 1). A series of trifluoromethane sulfonates, other Lewis acids, as well as some Brønsted acids were screened for this reaction. It was found that In(OTf)3 gave the best results based on the yields and chemoselectivity (Table 1, entries 2–13). Different solvents were employed for the further optimization of our reaction conditions (Table 2, entries 1–9). CH2Cl2, CHCl3, toluene and DCE were found to be a little superior to the other solutions, and CH2Cl2 was the optimal solvent (Table 2, entry 1).
Table 1 Evaluation of catalystsa
Table 2 Solution screeninga
The reaction conditions were further optimized by screening the catalyst loading, reaction times and molar ratios of 1a and 2a (Tables 3 and 4). It was found that the optimized model reaction conditions were a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 molar ratio of 1a and 2a in the presence of 15 mol% In(OTf)3 in CH2Cl2 for 10 hours, which could furnish 3a in 74% LC-yield (73% isolated yield) and 4a in 14% LC-yield, respectively (Table 4, entry 2).
1.5 molar ratio of 1a and 2a in the presence of 15 mol% In(OTf)3 in CH2Cl2 for 10 hours, which could furnish 3a in 74% LC-yield (73% isolated yield) and 4a in 14% LC-yield, respectively (Table 4, entry 2).
Table 3 Reaction optimizationa
Table 4 Effect of the ratioa
With the optimal reaction conditions in hand, we investigated the scope of the reaction utilizing a range of different 2-oxo-1-pyrrolidine derivatives and tryptamine derivatives. As shown in Table 5, all the reactions were completed with good generality. The substrates 2 bearing Ar with electron-donating groups, such as methyl and methoxy groups, could react well with N-tosyl tryptamine 1a to furnish the desired products in moderate yields with up to 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 chemoselectivity (Table 5, entries 1–5). The substrates 2 bearing Ar with strong electron-withdrawing groups, such as nitro and cyano groups, could proceed well to give the desired products in excellent yields with up to 85
5 chemoselectivity (Table 5, entries 1–5). The substrates 2 bearing Ar with strong electron-withdrawing groups, such as nitro and cyano groups, could proceed well to give the desired products in excellent yields with up to 85![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15 chemoselectivity (Table 5, entries 11 and 12). Some other substrates 2 bearing aryls, such as naphthalene-2-yl and thiophen-2-yl, were demonstrated to be good candidates for this reaction, giving high yields (Table 5, entries 13 and 14). As shown in Table 5, the substrates 2 bearing Ar with the same group at different positions gave similar results, reflecting that the steric effect has little influence (Table 5, entries 1–5, 7–10). As shown in Table 5, 2-substituted tryptamines were formed in a higher ratio compared to N-substituted tryptamines due to the steric congestion caused during the attack from different positions of the indole to the bulky iminium ion. The absolute configurations of 3k and 4f have been determined by X-ray diffraction (Scheme 4).
15 chemoselectivity (Table 5, entries 11 and 12). Some other substrates 2 bearing aryls, such as naphthalene-2-yl and thiophen-2-yl, were demonstrated to be good candidates for this reaction, giving high yields (Table 5, entries 13 and 14). As shown in Table 5, the substrates 2 bearing Ar with the same group at different positions gave similar results, reflecting that the steric effect has little influence (Table 5, entries 1–5, 7–10). As shown in Table 5, 2-substituted tryptamines were formed in a higher ratio compared to N-substituted tryptamines due to the steric congestion caused during the attack from different positions of the indole to the bulky iminium ion. The absolute configurations of 3k and 4f have been determined by X-ray diffraction (Scheme 4).
 |
| | Scheme 4 X-ray crystal structure of 3k and 4f. | |
Furthermore, tryptamine derivatives with different protecting groups were also explored (Table 6). All the reactions proceeded well to furnish the desired C-2 alkylation and N-alkylation products in moderate to excellent yields with high chemoselectivities (86![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 14). Unfortunately, it was found that no desired product was formed when tryptamine was used in the reaction, under identical reaction conditions.
14). Unfortunately, it was found that no desired product was formed when tryptamine was used in the reaction, under identical reaction conditions.
Based on the above results and ongoing efforts by our group,15 a plausible mechanism for this reaction is suggested in Scheme 5. The C–O bond of the 1-(ethoxy(4-nitrophenyl)methyl)pyrrolidin-2-one (2a) was activated in the presence of the Lewis acid to generate a stable intermediate of the iminium ion I and In(OTf)3(OEt)−. Under these circumstances, I was readily attacked by the C-2 position of the tryptamine derivative 1a (path [A]). The intermediate of the imine cation II was formed, followed by the elimination of a proton to afford the final product 3a. For the nucleophilicity of the N atom, the product 4a could also be obtained through the elimination of a proton from the intermediate III (path [B]). The proton was captured by the ethoxide ion of the In(OTf)3(OEt)− to regenerate EtOH and In(OTf)3. Because of the steric congestion caused during the attack from the different positions of the indole to the bulky iminium ion, 2-substituted tryptamines were formed in a higher ratio compared to N-substituted tryptamines.
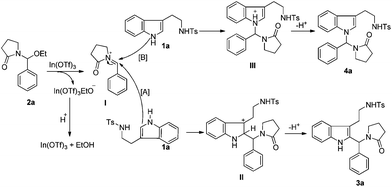 |
| | Scheme 5 Plausible mechanism. | |
Conclusions
In conclusion, we have developed a simple and highly efficient indium-catalyzed method to introduce various benzyl groups, as well as the 2-oxo-1-pyrrolidine moiety at the C-2 position of tryptamine derivatives, with good yields and chemoselectivity. In addition, our studies could not only enrich the library of Luzindole (3), but also provide a new way to synthesize a wide variety of potential pharmaceutically active compounds bearing a 2-oxo-1-pyrrolidine scaffold.
Experimental
General
Unless otherwise stated, all reagents were purchased from commercial suppliers and used without further purification. All reactions were carried out in air and using undistilled solvents, without any precautions to exclude air and moisture unless otherwise noted. Reactions were monitored by thin-layer chromatography (TLC) on silica gel GF-254 precoated glass plates. Chromatograms were visualized by fluorescence quenching with UV light at 254 nm. Flash column chromatography was performed using silica gel. Melting points were measured on a melting point apparatus and uncorrected. 1H and 13C NMR spectra were recorded in CDCl3 or D6-DMSO on 300 MHz or 400 MHz spectrometers. Tetramethylsilane (TMS) served as an internal standard for 1H NMR, and CDCl3 or D6-DMSO were used as internal standards for 13C NMR. IR spectra were recorded on a FT-IR spectrometer. Mass spectra were carried out using a quadrupole LC/MS system with an ESI source. HRMS was recorded on a commercial apparatus (ESI source).
General procedure for the In-catalyzed alkylation reaction
N-Tosyl tryptamine (0.5 mmol, 0.1575 g), 1-(ethoxy(phenyl)methyl) pyrrolidin-2-one (0.75 mmol, 0.1643 g), In(OTf)3 (0.075 mmol, 0.0421 g, 15 mol%), and CH2Cl2 (2 mL) were added into a flask. The mixture was then vigorously stirred at 40 °C until 1-phenylbutane-1,3-dione or 1-(ethoxy(phenyl)methyl)pyrrolidin-2-one was completely consumed, as monitored by TLC analysis. After the completion of the reaction, the residue was directly purified by flash column chromatography using ethyl acetate and petroleum ether as eluents to give the pure product.
4-Methyl-N-(2-(1-((2-oxopyrrolidin-1-yl)(phenyl)methyl)-1H-indol-3-yl)ethyl)benzenesulfonamide (4a). White solid (m.p. 70–71 °C), 14% yield (34.1 mg); 1H NMR (400 MHz, DMSO-d6) δ 7.66–7.61 (m, 3H), 7.57 (s, 1H), 7.48–7.40 (m, 4H), 7.35–7.27 (m, 3H), 7.19 (d, J = 7.1 Hz, 2H), 7.14 (t, J = 7.5 Hz, 1H), 7.07 (t, J = 7.4 Hz, 1H), 6.95 (s, 1H), 3.33–3.39 (m, 1H), 3.02–2.94 (m, 2H), 2.80–2.68 (m, 3H), 2.46–2.37 (m, 1H), 2.35 (s, 3H), 2.32–2.24 (m, 1H), 2.08–1.97 (m, 1H), 1.90–1.89 (m, 1H); 13C NMR (75 MHz, CDCl3) δ 175.63, 143.38, 137.00, 134.81, 129.65, 129.05, 128.78, 127.78, 126.95, 126.88, 124.72, 122.80, 120.38, 118.79, 111.79, 110.74, 63.72, 43.35, 43.08, 30.77, 25.67, 21.53, 18.00; IR (KBr) νmax: 3174, 2916, 1653, 1597, 1492, 1452, 1428, 1319, 1291, 1152, 1095, 925, 811, 744, 706, 664, 567, 545, 511 cm−1; HRMS (ESI): calcd for C28H29N3NaO3S: [M + Na]+ 510.1827, found: 510.1842%.
4-Methyl-N-(2-(2-((2-oxopyrrolidin-1-yl)(phenyl)methyl)-1H-indol-3-yl)ethyl)benzenesulfonamide (3a). White solid (85–86 °C), 74% yield (180.2 mg); 1H NMR (400 MHz, DMSO-d6) δ 10.70 (s, 1H), 7.62 (d, J = 8.2 Hz, 3H), 7.40–7.26 (m, 7H), 7.08–7.03 (m, 3H), 6.97 (t, J = 7.3 Hz, 1H), 6.56 (s, 1H), 3.46–3.40 (m, 1H), 3.02–3.10 (m, 1H), 2.90–2.73 (m, 4H), 2.43–2.36 (m, 1H), 2.34 (s, 3H), 2.30–2.20 (m, 1H), 2.06–1.97 (s, 1H), 1.94–1.85 (s, 1H); 13C NMR (75 MHz, CDCl3) δ 175.12, 142.07, 135.98, 135.67, 134.67, 131.59, 128.50, 127.81, 126.74, 126.14, 125.97, 125.72, 121.61, 118.76, 117.69, 110.33, 110.22, 50.89, 45.86, 42.26, 30.21, 23.58, 20.49, 17.25; IR (KBr) νmax: 3356, 3198, 2935, 1645, 1598, 1496, 1458, 1443, 1300, 1145, 1095, 931, 913, 812, 751, 709, 673, 574, 541, 530 cm−1; HRMS (ESI): calcd for C28H29N3NaO3S: [M + Na]+ 510.1827, found: 510.1823%.
N-(2-(1-((2-Methoxyphenyl)(2-oxopyrrolidin-1-yl)methyl)-1H-indol-3-yl)ethyl)-4-methylbenzenesulfonamide (4b). White solid (m.p. 30–31 °C), 11% yield (27.0 mg); 1H NMR (400 MHz, CDCl3) δ 7.68 (s, 1H), 7.56 (d, J = 7.3 Hz, 2H), 7.44–7.35 (m, 2H), 7.30 (d, J = 7.8 Hz, 1H), 7.22–7.17 (m, 1H), 7.14 (d, J = 7.4 Hz, 2H), 7.10–7.03 (m, 2H), 7.02–6.93 (m, 2H), 6.65 (s, 1H), 4.55 (s, 1H), 3.69 (s, 3H), 3.42–3.33 (m, 1H), 3.26–3.11 (m, 2H), 2.90–2.80 (m, 1H), 2.80–2.71 (m, 1H), 2.70–2.62 (m, 1H), 2.52–2.41 (m, 2H), 2.37 (s, 3H), 2.11–2.01 (m, 1H), 1.99–1.88 (m, 1H); 13C NMR (100 MHz, CDCl3) δ 175.63, 157.49, 143.45, 137.03, 136.53, 130.74, 129.81, 127.92, 127.19, 127.11, 124.23, 123.98, 122.70, 120.77, 120.12, 118.96, 111.37, 110.93, 110.62, 61.52, 55.90, 44.92, 43.09, 31.16, 25.66, 21.73, 18.51; IR (KBr) νmax: 3282, 2923, 1673, 1492, 1461, 1287, 1156, 1094, 742, 545, 535, 506 cm−1; HRMS (ESI): calcd for C29H31N3NaO4S: [M + Na]+ 540.1927, found: 540.1915%.
N-(2-(2-((2-Methoxyphenyl)(2-oxopyrrolidin-1-yl)methyl)-1H-indol-3-yl)ethyl)-4-methylbenzenesulfonamide (3b). White solid (m.p. 218–219 °C), 67% yield (173.3 mg); 1H NMR (400 MHz, CDCl3) δ 9.05 (s, 1H), 7.54 (d, J = 7.9 Hz, 2H), 7.37 (d, J = 7.8 Hz, 1H), 7.33–2.27 (m, 2H), 7.20–7.12 (m, 4H), 7.03 (t, J = 7.4 Hz, 1H), 6.91 (t, J = 7.0 Hz, 2H), 6.36 (s, 1H), 4.55 (s, 1H), 3.81 (s, 3H), 3.61–3.53 (m, 1H), 3.51–3.43 (m, 1H), 3.15 (s, 2H), 3.01–2.85 (m, 2H), 2.41 (t, J = 8.0 Hz, 2H), 2.37 (s, 3H), 2.10–1.96 (m, 2H); 13C NMR (75 MHz, CDCl3) δ 175.85, 156.69, 143.06, 136.61, 135.39, 133.67, 129.51, 129.38, 128.45, 127.31, 126.97, 126.06, 122.17, 120.55, 119.53, 118.42, 111.32, 110.83, 109.01, 55.63, 49.40, 48.55, 43.16, 31.53, 24.41, 21.50, 18.54; IR (KBr) νmax: 3259, 2937, 2855, 1655, 1490, 1460, 1438, 1324, 1244, 1161, 1106, 1020, 872, 816, 748, 551, 497 cm−1; HRMS (ESI): calcd for C29H31N3NaO4S: [M + Na]+ 540.1927, found: 540.1909%.
N-(2-(1-((3-Methoxyphenyl)(2-oxopyrrolidin-1-yl)methyl)-1H-indol-3-yl)ethyl)-4-methylbenzenesulfonamide (4c). White solid (m.p. 75–76 °C), 17% yield (45.8 mg); 1H NMR (400 MHz, CDCl3) δ 7.72 (s, 1H), 7.64 (d, J = 8.0 Hz, 2H), 7.49–7.42 (m, 2H), 7.35 (t, J = 8.0 Hz, 1H), 7.22 (d, J = 8.0 Hz, 3H), 7.14 (t, J = 7.4 Hz, 1H), 6.94 (d, J = 8.0 Hz, 1H), 6.80 (d, J = 7.6 Hz, 1H), 6.77–6.73 (m, 2H), 4.31 (s, 1H), 3.79 (s, 3H), 3.50–3.42 (m, 1H), 3.30–3.20 (m, 2H), 2.92–2.83 (m, 2H), 2.68–2.59 (m, 1H), 2.57–2.47 (m, 1H), 2.45–2.34 (m, 4H), 2.13–2.02 (m, 1H), 1.98–1.88 (m, 1H); 13C NMR (75 MHz, CDCl3) δ 175.62, 160.16, 143.35, 136.96, 136.51, 130.14, 129.65, 127.83, 126.95, 124.71, 122.77, 120.36, 119.11, 118.81, 113.72, 113.09, 111.86, 110.69, 63.65, 55.38, 43.42, 43.12, 30.75, 25.68, 21.52, 17.99; IR (KBr) νmax: 3529, 3147, 2941, 1675, 1595, 1492, 1462, 1329, 1225, 1158, 1094, 816, 745, 658, 550, 428 cm−1; HRMS (ESI): calcd for C29H31N3NaO4S: [M + Na]+ 540.1927, found: 540.1929%.
N-(2-(2-((3-Methoxyphenyl)(2-oxopyrrolidin-1-yl)methyl)-1H-indol-3-yl)ethyl)-4-methylbenzenesulfonamide (3c). White solid (m.p. 136–137 °C), 60% yield (153.1 mg); 1H NMR (400 MHz, CDCl3) δ 8.49 (s, 1H), 7.61 (d, J = 7.8 Hz, 2H), 7.47 (d, J = 7.8 Hz, 1H), 7.31–7.23 (m, 2H), 7.22–7.16 (m, 3H), 7.09 (t, J = 7.4 Hz, 1H), 6.83 (d, J = 8.0 Hz, 1H), 6.67 (d, J = 7.8 Hz, 1H), 6.65 (s, 1H), 6.44 (s, 1H), 4.76 (s, 1H), 3.75 (s, 3H), 3.67–3.59 (m, 1H), 3.41–3.33 (m, 1H), 3.20 (s, 2H), 3.08–2.91 (m, 2H), 2.49 (t, J = 8.0 Hz, 2H), 2.38 (s, 3H), 2.15–2.03 (m, 2H); 13C NMR (75 MHz, CDCl3) δ 175.02, 158.94, 142.03, 137.78, 135.73, 134.72, 131.27, 128.89, 128.50, 126.23, 125.96, 121.50, 118.66, 118.13, 117.71, 112.20, 111.42, 110.39, 110.26, 54.27, 50.61, 45.47, 42.37, 30.08, 23.60, 20.49, 17.18; IR (KBr) νmax: 3312, 3221, 2920, 1650, 1491, 1462, 1332, 1245, 1162, 1094, 1053, 874, 816, 766, 748, 549, 501 cm−1; HRMS (ESI): calcd for C29H31N3NaO4S: [M + Na]+ 540.1927, found: 540.1932%.
N-(2-(1-((4-Methoxyphenyl)(2-oxopyrrolidin-1-yl)methyl)-1H-indol-3-yl)ethyl)-4-methylbenzenesulfonamide (4d). White solid (m.p. 48–49 °C), 4% yield (10.3 mg); 1H NMR (400 MHz, CDCl3) δ 7.68 (s, 1H), 7.55 (d, J = 7.9 Hz, 2H), 7.45–7.35 (m, 2H), 7.32 (d, J = 8.2 Hz, 1H), 7.23–7.18 (m, 1H), 7.16 (d, J = 7.9 Hz, 2H), 7.12–7.05 (m, 2H), 7.03–6.95 (m, 2H), 6.63 (s, 1H), 4.21 (s, 1H), 3.71 (s, 3H), 3.45–3.36 (m, 1H), 3.29–3.14 (m, 2H), 2.91–2.83 (m, 1H), 2.80–2.72 (m, 1H), 2.70–2.62 (m, 1H), 2.51–2.41 (m, 2H), 2.38 (s, 3H), 2.12–2.02 (m, 1H), 1.98–1.90 (m, 1H); 13C NMR (75 MHz, CDCl3) δ 175.36, 157.29, 143.25, 136.79, 136.35, 130.53, 129.59, 127.66, 126.89, 124.05, 123.75, 122.52, 120.55, 119.93, 118.70, 111.18, 110.61, 61.31, 55.68, 44.67, 42.79, 30.93, 25.42, 21.50, 18.29; IR (KBr) νmax: 3150, 2928, 1670, 1598, 1490, 1457, 1411, 1328, 1267, 1159, 1089, 1016, 712, 698, 635, 576, 544 cm−1; HRMS (ESI): calcd for C29H31N3NaO4S: [M + Na]+ 540.1927, found: 540.1922%.
N-(2-(2-((4-Methoxyphenyl)(2-oxopyrrolidin-1-yl)methyl)-1H-indol-3-yl)ethyl)-4-methylbenzenesulfonamide (3d). White solid (m.p. 203–204 °C), 70% yield (182.8 mg); 1H NMR (400 MHz, CDCl3) δ 9.06 (s, 1H), 7.55 (d, J = 7.8 Hz, 2H), 7.37 (d, J = 7.8 Hz, 1H), 7.34–7.27 (m, 2H), 7.20–7.13 (m, 4H), 7.03 (t, J = 7.4 Hz, 1H), 6.91 (t, J = 6.9 Hz, 2H), 6.36 (s, 1H), 4.58 (s, 1H), 3.81 (s, 3H), 3.61–3.53 (m, 1H), 3.51–3.42 (m, 1H), 3.15 (s, 2H), 3.01–2.85 (m, 2H), 2.42 (t, J = 7.9 Hz, 2H), 2.37 (s, 3H), 2.10–1.96 (m, 2H); 13C NMR (75 MHz, DMSO-d6) δ 174.17, 156.97, 142.94, 138.00, 135.98, 133.43, 130.02, 129.39, 127.99, 126.99, 121.42, 120.79, 119.04, 118.19, 111.86, 111.37, 108.91, 55.86, 47.40, 46.12, 43.60, 30.79, 24.86, 21.37, 18.15; IR (KBr) νmax: 3424, 3215, 2964, 2916, 2849, 1669, 1598, 1493, 1461, 1421, 1324, 1160, 1105, 1022, 934, 912, 817, 800, 751, 739, 653, 572, 551, 498 cm−1; HRMS (ESI): calcd for C29H31N3NaO4S: [M + Na]+ 540.1927, found: 540.1932%.
4-Methyl-N-(2-(1-((2-oxopyrrolidin-1-yl)(p-tolyl)methyl)-1H-indol-3-yl)ethyl)benzenesulfonamide (4e). White solid (m.p. 37–38), 14% yield (21.5 mg); 1H NMR (400 MHz, CDCl3) δ 7.72 (s, 1H), 7.64 (d, J = 7.9 Hz, 2H), 7.45 (t, J = 7.9 Hz, 2H), 7.23 (t, J = 8.2 Hz, 5H), 7.16–7.11 (m, 1H), 7.09 (d, J = 7.8 Hz, 2H), 6.75 (s, 1H), 4.28 (s, 1H), 3.45 (t, J = 7.4 Hz, 1H), 3.30–3.19 (m, J = 6.3 Hz, 2H), 2.91–2.82 (m, 2H), 2.70–2.61 (m, 1H), 2.58–2.48 (m, 1H), 2.46–2.34 (m, 7H), 2.15–2.03 (m, 1H), 1.99–1.88 (m, 1H); 13C NMR (100 MHz, CDCl3) δ 175.90, 143.50, 138.83, 137.26, 137.14, 132.01, 129.93, 129.86, 128.13, 127.18, 127.06, 124.88, 122.88, 120.49, 119.05, 112.07, 110.93, 63.90, 43.62, 43.44, 31.05, 25.91, 21.76, 21.39, 18.21; IR (KBr) νmax: 3183, 2923, 1652, 1458, 1327, 1158, 1095, 780, 741, 545, 495 cm−1; HRMS (ESI): calcd for C29H31N3NaO3S: [M + Na]+ 524.1978, found: 524.1970%.
4-Methyl-N-(2-(2-((2-oxopyrrolidin-1-yl)(p-tolyl)methyl)-1H-indol-3-yl)ethyl)benzenesulfonamide (3e). White solid (m.p. 157–158), 63% yield (95.1 mg); 1H NMR (400 MHz, CDCl3) δ 8.55 (s, 1H), 7.60 (d, J = 7.9 Hz, 2H), 7.46 (d, J = 7.8 Hz, 1H), 7.28 (d, J = 8.3 Hz, 1H), 7.18 (d, J = 7.5 Hz, 3H), 7.13 (d, J = 7.7 Hz, 2H), 7.08 (t, J = 7.4 Hz, 1H), 6.97 (d, J = 7.7 Hz, 2H), 6.41 (s, 1H), 4.80 (s, 1H), 3.67–3.58 (m, 1H), 3.44–3.35 (m, 1H), 3.19 (s, 2H), 3.07–2.92 (m, 2H), 2.49 (t, J = 7.9 Hz, 2H), 2.37 (s, 3H), 2.34 (s, 3H), 2.13–2.03 (m, 2H); 13C NMR (75 MHz, CDCl3) δ 175.07, 142.04, 136.49, 135.71, 134.63, 132.90, 131.81, 128.49, 126.19, 125.97, 125.69, 121.52, 118.71, 117.67, 110.30, 110.02, 50.73, 45.83, 42.28, 30.25, 23.56, 20.49, 20.05, 17.24; IR (KBr) νmax: 3390, 3085, 2919, 1659, 1514, 1493, 1461, 1328, 1295, 1211, 1159, 1073, 913, 823, 747, 655, 573 cm−1; HRMS (ESI): calcd for C29H31N3NaO3S: [M + Na]+ 524.1978, found: 524.1974%.
4-Methyl-N-(2-(1-((2-oxopyrrolidin-1-yl)(o-tolyl)methyl)-1H-indol-3-yl)ethyl)benzenesulfonamide (4f). White solid (m.p. 47–48 °C), 26% yield (65.4 mg); 1H NMR (400 MHz, CDCl3) δ 7.62 (d, J = 8.1 Hz, 2H), 7.58 (s, 1H), 7.44 (d, J = 7.9 Hz, 1H), 7.36–7.29 (m, 2H), 7.28–7.27 (m, 1H), 7.25–7.20 (m, 3H), 7.20–7.15 (m, 2H), 7.13 (t, J = 7.5 Hz, 1H), 6.53 (s, 1H), 4.39 (s, 1H), 3.48–3.41 (m, 1H), 3.24–3.16 (m, 2H), 2.81 (t, J = 6.9 Hz, 2H), 2.59–2.42 (m, 3H), 2.39 (s, 3H), 2.17–2.04 (m, 1H), 1.99–1.90 (m, 4H); 13C NMR (75 MHz, CDCl3) δ 175.34, 143.35, 137.07, 136.20, 133.68, 131.55, 129.65, 129.07, 127.80, 126.93, 126.32, 125.59, 123.76, 122.92, 120.40, 118.95, 111.76, 110.18, 76.60, 62.98, 44.36, 43.13, 30.84, 25.61, 21.52, 18.86, 18.16; IR (KBr) νmax: 3488, 2923, 2134, 1670, 1459, 1414, 1327, 1287, 1266, 1155, 1094, 818, 745, 686, 661, 552 cm−1; HRMS (ESI): calcd for C29H31N3NaO3S: [M + Na]+ 524.1984, found: 524.1979%.
4-Methyl-N-(2-(2-((2-oxopyrrolidin-1-yl)(o-tolyl)methyl)-1H-indol-3-yl)ethyl)benzenesulfonamide (3f). White solid (m.p. 169–170 °C), 54% yield (134.5 mg); 1H NMR (400 MHz, CDCl3) δ 8.05 (s, 1H), 7.64 (d, J = 7.6 Hz, 2H), 7.44 (d, J = 7.5 Hz, 1H), 7.26–7.19 (m, 5H), 7.18–7.11 (m, 3H), 7.11–7.04 (m, 1H), 6.57 (s, 1H), 5.02 (s, 1H), 3.51–3.39 (m, 1H), 3.21–3.01 (m, 3H), 2.95–2.83 (m, 2H), 2.51–2.42 (m, 2H), 2.39 (s, 3H), 2.06 (s, 3H), 2.04–1.96 (m, 1H), 1.84 (s, 1H); 13C NMR (75 MHz, CDCl3) δ 175.43, 143.12, 136.79, 136.56, 135.75, 135.38, 132.02, 131.33, 129.56, 128.14, 127.77, 127.07, 126.35, 126.06, 122.40, 119.89, 118.62, 111.25, 110.51, 50.41, 46.66, 43.25, 30.99, 24.63, 21.51, 19.36, 18.20; IR (KBr) νmax: 3428, 3188, 2918, 1653, 1457, 1433, 1307, 1322, 1289, 1158, 1093, 748, 720, 667, 550, 484, 456 cm−1; HRMS (ESI): calcd for C29H31N3NaO3S: [M + Na]+ 524.1984, found: 524.1971%.
N-(2-(1-((4-Fluorophenyl)(2-oxopyrrolidin-1-yl)methyl)-1H-indol-3-yl)ethyl)-4-methylbenzenesulfonamide (4g). White solid (m.p. 144–145 °C), 16% yield (40.5 mg); 1H NMR (400 MHz, CDCl3) δ 7.72 (s, 1H), 7.65 (d, J = 7.8 Hz, 2H), 7.48–7.40 (m, 2H), 7.23 (d, J = 7.6 Hz, 3H), 7.21–7.16 (m, 2H), 7.16–7.07 (m, 3H), 6.74 (s, 1H), 4.32 (s, 1H), 3.47–3.39 (m, 1H), 3.30–3.21 (m, 2H), 2.89 (t, J = 6.6 Hz, 2H), 2.73–2.62 (m, 1H), 2.58–2.48 (m, 1H), 2.46–2.35 (m, 4H), 2.14–2.02 (m, 1H), 2.01–1.90 (m, 1H); 13C NMR (75 MHz, CDCl3) δ 175.70, 143.38, 136.99, 130.70, 129.65, 128.81, 128.70, 127.90, 126.94, 124.44, 122.84, 120.44, 118.88, 116.19, 115.90, 112.20, 110.66, 63.37, 43.32, 43.11, 30.72, 25.69, 21.51, 17.98; IR (KBr) νmax: 3405, 2921, 1667, 1508, 1459, 1327, 1232, 1159, 1087, 744, 550, 457, 418 cm−1; HRMS (ESI): calcd for C28H28FN3NaO3S: [M + Na]+ 528.1728, found: 528.1711%.
N-(2-(2-((4-Fluorophenyl)(2-oxopyrrolidin-1-yl)methyl)-1H-indol-3-yl)ethyl)-4-methylbenzenesulfonamide (3g). White solid (m.p. 167–16), 60% yield (150.5 mg); 1H NMR (400 MHz, CDCl3) δ 8.70 (s, 1H), 7.60 (d, J = 7.6 Hz, 2H), 7.46 (d, J = 7.7 Hz, 1H), 7.30 (d, J = 8.0 Hz, 1H), 7.19 (d, J = 7.4 Hz, 3H), 7.13–7.03 (m, 3H), 7.03–6.95 (m, 2H), 6.35 (s, 1H), 4.75 (s, 1H), 3.69–3.58 (m, 1H), 3.50–3.40 (m, 1H), 3.18 (s, 2H), 3.09–2.92 (m, 2H), 2.54–2.45 (m, 2H), 2.38 (s, 3H), 2.15–2.04 (m, 2H); 13C NMR (75 MHz, CDCl3) δ 176.20, 143.15, 136.69, 135.81, 132.96, 132.31, 129.54, 128.62, 128.51, 126.95, 122.65, 119.76, 118.71, 115.79, 115.50, 111.47, 111.24, 51.56, 46.88, 43.40, 31.20, 24.66, 21.50, 18.24; IR (KBr) νmax: 3371, 3189, 2933, 1644, 1599, 1512, 1459, 1300, 1233, 1146, 1095, 869, 812, 746, 672, 573, 549 cm−1; HRMS (ESI): calcd for C28H28FN3NaO3S: [M + Na]+ 528.1728, found: 528.1712%.
N-(2-(1-((4-Bromophenyl)(2-oxopyrrolidin-1-yl)methyl)-1H-indol-3-yl)ethyl)-4-methylbenzenesulfonamide (4h). White solid (m.p. 77–78 °C), 15% yield (41.2 mg); 1H NMR (400 MHz, CDCl3) δ 7.69 (s, 1H), 7.65 (d, J = 7.8 Hz, 2H), 7.56 (d, J = 8.1 Hz, 2H), 7.48–7.40 (m, 2H), 7.24 (d, J = 7.4 Hz, 3H), 7.15 (t, J = 7.1 Hz, 1H), 7.09 (d, J = 7.8 Hz, 2H), 6.72 (s, 1H), 4.33 (s, 1H), 3.41 (s, 1H), 3.29–3.21 (m, 2H), 2.92–2.95 (m, 2H), 2.72–2.63 (m, 1H), 2.58–2.48 (m, 1H), 2.46–2.35 (m, 4H), 2.15–2.02 (m, 1H), 2.01–1.90 (m, 1H); 13C NMR (75 MHz, CDCl3) δ 175.70, 143.40, 136.97, 136.88, 134.07, 132.21, 129.66, 128.65, 127.86, 126.94, 124.41, 122.92, 120.50, 118.89, 112.29, 110.65, 63.40, 43.31, 43.09, 30.67, 25.68, 21.53, 17.98; IR (KBr) νmax: 3442, 3179, 1663, 1458, 1416, 1328, 1263, 1157, 1088, 813, 801, 745, 548, 428, 420 cm−1; HRMS (ESI): calcd for C28H2881BrN3NaO3S: [M + Na]+ 590.0912, found: 590.0917%.
N-(2-(2-((4-Bromophenyl)(2-oxopyrrolidin-1-yl)methyl)-1H-indol-3-yl)ethyl)-4-methylbenzenesulfonamide (3h). White solid (m.p. 204–205 °C), 83% yield (235.2 mg); 1H NMR (400 MHz, DMSO-d6) δ 10.70 (s, 1H), 7.63 (d, J = 7.9 Hz, 3H), 7.55 (d, J = 8.1 Hz, 2H), 7.40 (d, J = 7.8 Hz, 1H), 7.33 (d, J = 7.8 Hz, 2H), 7.28 (d, J = 8.0 Hz, 1H), 7.11–6.94 (m, 4H), 6.53 (s, 1H), 3.45–3.38 (m, 1H), 3.05–2.96 (m, 7.9 Hz, 1H), 2.89–2.75 (m, 4H), 2.44–2.36 (m, 1H), 2.35 (s, 3H), 2.30–2.17 (m, 1H), 2.07–1.95 (m, 1H), 1.95–1.82 (m, 1H); 13C NMR (75 MHz, DMSO-d6) δ 173.61, 141.89, 137.00, 136.96, 135.42, 131.22, 130.83, 128.95, 128.52, 126.45, 125.97, 121.00, 119.89, 118.18, 117.74, 110.93, 110.08, 49.07, 43.83, 43.01, 29.63, 23.88, 20.37, 17.05; IR (KBr) νmax: 3351, 3196, 2923, 1648, 1489, 1436, 1309, 1145, 1094, 1010, 929, 913, 812, 748, 670, 542, 521 cm−1; HRMS (ESI): calcd for C28H2881BrN3NaO3S: [M + Na]+ 590.0912, found: 590.0914%.
N-(2-(1-((4-Chlorophenyl)(2-oxopyrrolidin-1-yl)methyl)-1H-indol-3-yl)ethyl)-4-methylbenzenesulfonamide (4i). White solid (m.p. 52–53 °C), 15% yield (38.4 mg); 1H NMR (400 MHz, CDCl3) δ 7.71 (s, 1H), 7.65 (d, J = 7.8 Hz, 2H), 7.49–7.42 (m, 2H), 7.41 (d, J = 7.5 Hz, 2H), 7.23 (d, J = 7.5 Hz, 3H), 7.15 (d, J = 7.4 Hz, 3H), 6.73 (s, 1H), 4.33 (s, 1H), 3.42 (t, J = 7.8 Hz, 1H), 3.31–3.19 (m, 2H), 2.89 (t, J = 6.4 Hz, 2H), 2.73–2.63 (m, 1H), 2.59–2.48 (m, 1H), 2.45–2.35 (m, 4H), 2.15–2.02 (m, 1H), 2.00–1.90 (m, 1H); 13C NMR (75 MHz, CDCl3) δ 175.71, 143.40, 136.98, 136.88, 134.76, 133.51, 129.65, 129.25, 128.34, 127.88, 126.93, 124.41, 122.89, 120.49, 118.89, 112.29, 110.65, 63.37, 43.33, 43.09, 30.67, 25.68, 21.52, 17.98; IR (KBr) νmax: 3414, 3188, 2925, 1664, 1492, 1459, 1329, 1286, 1158, 1089, 1011, 815, 745, 544 cm−1; HRMS (ESI): calcd for C28H28ClN3NaO3S: [M + Na]+ 544.1432, found: 544.1431%.
N-(2-(2-((4-Chlorophenyl)(2-oxopyrrolidin-1-yl)methyl)-1H-indol-3-yl)ethyl)-4-methylbenzenesulfonamide (3i). White solid (m.p. 216–217), 70% yield (182.8 mg); 1H NMR (400 MHz, CDCl3) δ 8.71 (s, 1H), 7.60 (d, J = 8.0 Hz, 2H), 7.47 (d, J = 7.9 Hz, 1H), 7.29 (t, J = 8.0 Hz, 3H), 7.20 (t, J = 6.9 Hz, 3H), 7.09 (t, J = 7.4 Hz, 1H), 7.02 (d, J = 8.2 Hz, 2H), 6.32 (s, 1H), 4.70 (s, 1H), 3.70–3.61 (m, 1H), 3.51–3.43 (m, 1H), 3.24–3.12 (m, 2H), 3.10–2.92 (m, 2H), 2.55–2.44 (m, 2H), 2.38 (s, 3H), 2.18–2.07 (m, 2H); 13C NMR (75 MHz, CDCl3) δ 176.29, 143.22, 136.64, 135.79, 135.74, 133.65, 132.29, 129.57, 128.95, 128.13, 126.98, 122.84, 119.89, 118.69, 111.44, 111.26, 51.99, 47.50, 43.26, 31.28, 24.73, 21.50, 18.34; IR (KBr) νmax: 3320, 3212, 2922, 1667, 1598, 1490, 1461, 1437, 1334, 1307, 1161, 1090, 1058, 1014, 871, 804, 756, 742, 659, 573, 548, 499 cm−1; HRMS (ESI): calcd for C28H28ClN3NaO3S: [M + Na]+ 544.1432, found: 544.1434%.
N-(2-(1-((3-Chlorophenyl)(2-oxopyrrolidin-1-yl)methyl)-1H-indol-3-yl)ethyl)-4-methylbenzenesulfonamide (4j). White solid (m.p. 57–58 °C), 22% yield (58.0 mg); 1H NMR (400 MHz, CDCl3) δ 7.71 (s, 1H), 7.65 (d, J = 7.7 Hz, 2H), 7.49–7.41 (m, 2H), 7.40–7.33 (m, 2H), 7.26–7.17 (m, 4H), 7.15 (t, J = 7.4 Hz, 1H), 7.09 (d, J = 6.5 Hz, 1H), 6.72 (s, 1H), 4.44 (s, 1H), 3.43 (t, J = 7.9 Hz, 1H), 3.25 (s, 2H), 2.89 (t, J = 6.5 Hz, 2H), 2.72–2.62 (m, 1H), 2.61–2.48 (m, 1H), 2.46–2.33 (m, 4H), 2.18–2.04 (m, 1H), 2.01–1.89 (m, 1H); 13C NMR (75 MHz, CDCl3) δ 175.78, 143.36, 137.21, 137.00, 136.91, 135.15, 130.40, 129.66, 129.03, 127.88, 126.94, 125.26, 124.38, 122.92, 120.51, 118.93, 112.47, 110.58, 63.27, 43.34, 43.14, 30.64, 25.69, 21.52, 17.97; IR (KBr) νmax: 3463, 2928, 2880, 1739, 1654, 1599, 1490, 1458, 1384, 1234, 1217, 1155, 1105, 1093, 1020, 743, 667, 582, 548, 470 cm−1; HRMS (ESI): calcd for C28H28ClN3NaO3S: [M + Na]+ 544.1432, found: 544.1431%.
N-(2-(2-((3-Chlorophenyl)(2-oxopyrrolidin-1-yl)methyl)-1H-indol-3-yl)ethyl)-4-methylbenzenesulfonamide (3j). White solid (m.p. 74–75 °C), 75% yield (194.3 mg); 1H NMR (400 MHz, CDCl3) δ 8.70 (s, 1H), 7.60 (d, J = 7.5 Hz, 2H), 7.47 (d, J = 7.8 Hz, 1H), 7.33–7.26 (m, 2H), 7.25–7.15 (m, 4H), 7.14–7.03 (m, 2H), 6.97 (d, J = 6.0 Hz, 1H), 6.35 (s, 1H), 4.77 (s, 1H), 3.69–3.59 (m, 1H), 3.49–3.39 (m, 1H), 3.23–3.13 (m, 2H), 3.10–2.90 (m, 2H), 2.49 (t, J = 7.8 Hz, 2H), 2.38 (s, 3H), 2.16–2.06 (m, 2H); 13C NMR (75 MHz, CDCl3) δ 176.21, 143.14, 139.57, 136.75, 135.89, 134.81, 131.78, 130.08, 129.55, 127.93, 127.09, 126.95, 126.83, 125.09, 122.75, 119.80, 118.76, 111.53, 51.71, 46.95, 43.40, 31.10, 24.74, 21.49, 18.24; IR (KBr) νmax: 3344, 2926, 1665, 1596, 1459, 1420, 1325, 1157, 1094, 890, 814, 745, 659, 550 cm−1; HRMS (ESI): calcd for C28H28ClN3NaO3S: [M + Na]+ 544.1432, found: 544.1433%.
N-(2-(1-((2-Bromophenyl)(2-oxopyrrolidin-1-yl)methyl)-1H-indol-3-yl)ethyl)-4-methylbenzenesulfonamide (4k). White solid (m.p. 44–45 °C), 10% yield (28.8 mg); 1H NMR (400 MHz, CDCl3) δ 7.66 (d, J = 7.8 Hz, 1H), 7.62 (d, J = 7.8 Hz, 2H), 7.55 (s, 1H), 7.42 (d, J = 7.7 Hz, 1H), 7.36 (t, J = 7.6 Hz, 1H), 7.32–7.26 (m, 2H), 7.23–7.17 (m, 3H), 7.13–7.07 (m, 2H), 6.62 (s, 1H), 4.51 (t, J = 6.0 Hz, 1H), 3.39–3.31 (m, 1H), 3.28–3.15 (m, 2H), 2.93–2.75 (m, 3H), 2.57–2.44 (m, 2H), 2.38 (s, 3H), 2.16–2.07 (m, 1H), 2.05–1.98 (m, 1H); 13C NMR (75 MHz, CDCl3) δ 175.52, 143.27, 136.95, 136.22, 135.09, 134.05, 130.62, 129.63, 127.90, 127.75, 126.91, 123.64, 123.48, 122.82, 120.29, 118.90, 111.92, 110.26, 65.20, 44.83, 43.00, 30.74, 25.57, 21.52, 18.32; IR (KBr) νmax: 3413, 2919, 1679, 1460, 1411, 1328, 1285, 1156, 1093, 1018, 812, 749, 669, 551, 446 cm−1; HRMS (ESI): calcd for C28H28BrN3NaO3S: [M + Na]+ 588.0932, found: 588.0934%.
N-(2-(2-((2-Bromophenyl)(2-oxopyrrolidin-1-yl)methyl)-1H-indol-3-yl)ethyl)-4-methylbenzenesulfonamide (3k). White solid (m.p. 165–166), 76% yield (214.3 mg); 1H NMR (400 MHz, CDCl3) δ 8.69 (s, 1H), 7.60 (d, J = 7.7 Hz, 2H), 7.56 (d, J = 7.9 Hz, 1H), 7.42 (d, J = 7.8 Hz, 1H), 7.31–7.25 (m, 2H), 7.20–7.12 (m, 4H), 7.06 (t, J = 7.3 Hz, 1H), 6.35 (s, 1H), 4.81 (s, 1H), 3.43 (t, J = 6.6 Hz, 2H), 3.18–3.06 (m, 2H), 2.96–2.83 (m, 2H), 2.42 (t, J = 7.9 Hz, 2H), 2.38 (s, 3H), 2.09–1.99 (m, 2H); 13C NMR (75 MHz, CDCl3) δ 175.99, 143.19, 137.15, 136.63, 135.60, 133.65, 132.13, 129.71, 129.59, 129.32, 127.70, 127.05, 123.54, 122.63, 119.92, 118.66, 111.39, 110.38, 53.94, 48.26, 43.08, 31.26, 24.67, 21.53, 18.49; IR (KBr) νmax: 3362, 2924, 1668, 1597, 1493, 1461, 1417, 1324, 1289, 1157, 1093, 1020, 814, 746, 661, 550, 435 cm−1; HRMS (ESI): calcd for C28H28BrN3NaO3S: [M + Na]+ 588.0932, found: 588.0925%.
N-(2-(1-((4-Cyanophenyl)(2-oxopyrrolidin-1-yl)methyl)-1H-indol-3-yl)ethyl)-4-methylbenzenesulfonamide (4l). White solid (m.p. 84–85 °C), 27% yield (69.6 mg); 1H NMR (400 MHz, CDCl3) δ 7.76 (s, 1H), 7.71 (d, J = 7.3 Hz, 2H), 7.65 (d, J = 7.8 Hz, 2H), 7.47 (d, J = 8.0 Hz, 1H), 7.39 (d, J = 8.1 Hz, 1H), 7.34 (d, J = 7.5 Hz, 2H), 7.25–7.19 (m, 3H), 7.15 (t, J = 6.9 Hz, 1H), 6.73 (s, 1H), 4.73 (t, J = 5.2 Hz, 1H), 3.40 (t, J = 8.7 Hz, 1H), 3.28–3.18 (m, 2H), 2.90 (t, J = 6.5 Hz, 2H), 2.78–2.69 (m, 1H), 2.58–2.49 (m, 1H), 2.45–2.35 (m, 4H), 2.15–2.03 (m, 1H), 2.03–1.90 (m, 1H); 13C NMR (75 MHz, CDCl3) δ 175.92, 143.43, 140.52, 136.96, 136.85, 132.84, 129.67, 127.80, 126.90, 124.27, 123.03, 120.64, 119.02, 118.23, 112.96, 112.76, 110.49, 63.48, 43.40, 43.05, 30.54, 25.67, 21.52, 17.96; IR (KBr) νmax: 3277, 2925, 2853, 2229, 1677, 1598, 1507, 1460, 1412, 1326, 1158, 1094, 1017, 880, 814, 744, 660, 550, 427 cm−1; HRMS (ESI): calcd for C29H28N4NaO3S: [M + Na]+ 535.1780, found: 535.1783%.
N-(2-(2-((4-Cyanophenyl)(2-oxopyrrolidin-1-yl)methyl)-1H-indol-3-yl)ethyl)-4-methylbenzenesulfonamide (3l). White solid (m.p. 245–246 °C), 68% yield (176.2 mg); 1H NMR (400 MHz, CDCl3) δ 8.94 (s, 1H), 7.63–7.54 (m, 4H), 7.50–7.42 (m, 1H), 7.35–7.27 (m, 1H), 7.24–7.16 (m, 5H), 7.14–7.06 (m, 1H), 6.26 (s, 1H), 4.60 (s, 1H), 3.79–3.68 (m, 1H), 3.63–3.52 (m, 1H), 3.25–3.13 (m, 2H), 3.11–2.94 (m, 2H), 2.58–2.44 (m, 2H), 2.40 (s, 3H), 2.22–2.10 (m, 2H); 13C NMR (75 MHz, CDCl3) δ 176.61, 143.42, 143.06, 136.52, 135.88, 132.53, 131.76, 129.64, 127.44, 126.98, 126.68, 123.13, 120.00, 118.68, 111.61, 111.55, 52.89, 48.38, 43.20, 31.28, 24.92, 21.51, 18.46; IR (KBr) νmax: 3247, 2914, 2231, 1664, 1605, 1493, 1459, 1332, 1268, 1162, 1059, 872, 763, 746, 659, 562, 550 cm−1; HRMS (ESI): calcd for C29H28N4NaO3S: [M + Na]+ 535.1780, found: 535.1765%.
4-Methyl-N-(2-(1-((4-nitrophenyl)(2-oxopyrrolidin-1-yl)methyl)-1H-indol-3-yl)ethyl)benzenesulfonamide (4m). White solid (m.p. 45–46 °C), 15% yield (39.6 mg); 1H NMR (400 MHz, CDCl3) δ 8.29 (d, J = 8.5 Hz, 2H), 7.81 (s, 1H), 7.66 (d, J = 8.0 Hz, 2H), 7.49 (d, J = 7.7 Hz, 1H), 7.41 (d, J = 8.1 Hz, 3H), 7.26–7.22 (m, 3H), 7.17 (t, J = 7.4 Hz, 1H), 6.74 (s, 1H), 4.34 (s, 1H), 3.47–3.39 (m, 1H), 3.30–3.21 (m, 2H), 2.92 (t, J = 6.5 Hz, 2H), 2.80–2.71 (m, 1H), 2.62–2.52 (m, 1H), 2.48–2.37 (m, 4H), 2.20–2.08 (m, 1H), 2.05–1.96 (m, 1H); 13C NMR (75 MHz, CDCl3) δ 175.93, 148.10, 143.47, 142.41, 136.91, 136.84, 129.68, 128.06, 127.93, 126.90, 124.22, 123.10, 120.71, 119.05, 113.06, 110.50, 63.42, 43.43, 43.05, 30.52, 25.68, 21.51, 17.99; IR (KBr) νmax: 3417, 3164, 2925, 1667, 1598, 1519, 1458, 1412, 1349, 1264, 1158, 1084, 1016, 811, 800, 748, 628, 552 cm−1; HRMS (ESI): calcd for C28H28N4NaO5S: [M + Na]+ 555.1673, found: 555.1670%.
4-Methyl-N-(2-(2-((4-nitrophenyl)(2-oxopyrrolidin-1-yl)methyl)-1H-indol-3-yl)ethyl)benzenesulfonamide (3m). White solid (m.p. 107–108 °C), 84% yield (222.8 mg); 1H NMR (400 MHz, CDCl3) δ 9.00 (s, 1H), 8.14 (d, J = 7.8 Hz, 2H), 7.60 (d, J = 7.4 Hz, 2H), 7.48 (d, J = 7.5 Hz, 1H), 7.31 (t, J = 11.0 Hz, 1H), 7.26–7.15 (m, 5H), 7.15–7.05 (m, 1H), 6.28 (s, 1H), 4.63 (s, 1H), 3.82–3.69 (m, 1H), 3.68–3.54 (m, 1H), 3.16 (s, 2H), 3.12–2.94 (m, 2H), 2.60–2.44 (m, 2H), 2.39 (s, 3H), 2.24–2.08 (m, 2H); 13C NMR (75 MHz, CDCl3) δ 176.65, 147.26, 145.11, 143.46, 136.45, 135.92, 131.69, 129.64, 127.63, 126.97, 126.67, 123.89, 123.15, 120.00, 118.70, 111.65, 111.58, 52.81, 48.40, 43.25, 31.27, 24.93, 21.51, 18.47; IR (KBr) νmax: 3317, 3242, 2915, 1671, 1597, 1517, 1459, 1433 1356, 1162, 1084, 1014, 877, 766, 749, 659, 547 cm−1; HRMS (ESI): calcd for C28H28N4NaO5S: [M + Na]+ 555.1673, found: 555.1671%.
4-Methyl-N-(2-(1-(naphthalen-1-yl(2-oxopyrrolidin-1-yl)methyl)-1H-indol-3-yl)ethyl)benzenesulfonamide (4n). White solid (m.p. 72–73 °C), 16% yield (43.3 mg); 1H NMR (400 MHz, CDCl3) δ 7.92–2.87 (m, 3H), 7.87–7.82 (m, 1H), 7.69 (s, 1H), 7.62 (d, J = 8.0 Hz, 2H), 7.58–7.53 (m, 2H), 7.48 (t, J = 7.4 Hz, 2H), 7.29–7.22 (m, 2H), 7.15 (t, J = 7.4 Hz, 3H), 6.72 (s, 1H), 4.32 (s, 1H), 3.58–3.50 (m, 1H), 3.29–3.22 (m, 2H), 2.86 (t, J = 6.5 Hz, 2H), 2.75–2.66 (m, 1H), 2.64–2.55 (m, 1H), 2.50–2.40 (m, 1H), 2.35 (s, 3H), 2.21–2.12 (m, 1H), 2.03–1.94 (m, 1H); 13C NMR (75 MHz, CDCl3) δ 175.76, 143.32, 137.02, 136.95, 133.24, 133.09, 132.29, 129.61, 129.07, 128.20, 127.86, 127.75, 126.92, 126.87, 126.77, 125.83, 124.76, 124.56, 122.87, 120.44, 118.87, 111.92, 110.75, 64.00, 43.54, 43.05, 30.83, 25.63, 21.46, 18.08; IR (KBr) νmax: 3277, 2923, 1670, 1459, 1328, 1157, 1093, 821, 746, 661, 554, 476 cm−1; HRMS (ESI): calcd for C32H31N3NaO3S: [M + Na]+ 560.1978, found: 560.1975%.
4-Methyl-N-(2-(2-(naphthalen-1-yl(2-oxopyrrolidin-1-yl)methyl)-1H-indol-3-yl)ethyl)benzenesulfonamide (3n). White solid (m.p. 193–194 °C), 71% yield (190.2 mg); 1H NMR (400 MHz, CDCl3) δ 8.95 (s, 1H), 7.60 (d, J = 7.6 Hz, 4H), 7.47 (d, J = 7.8 Hz, 1H), 7.32 (d, J = 8.0 Hz, 1H), 7.26–7.17 (m, 8H), 7.11 (t, J = 7.4 Hz, 1H), 6.27 (s, 1H), 4.61 (s, 1H), 3.79–3.71 (m, 1H), 3.62–3.55 (m, 1H), 3.20 (s, 2H), 3.13–2.97 (m, 2H), 2.57–2.47 (m, 2H), 2.40 (s, 3H), 2.21–2.12 (m, 2H); 13C NMR (75 MHz, CDCl3) δ 175.56, 142.39, 142.07, 135.47, 134.86, 131.49, 130.70, 128.61, 126.45, 125.95, 125.66, 122.07, 118.95, 117.66, 117.45, 110.59, 110.53, 51.80, 47.29, 42.20, 30.27, 23.87, 20.50, 17.36; IR (KBr) νmax: 3322, 3209, 2923, 1655, 1489, 1464, 1435, 1328, 1151, 1091, 818, 751, 657, 565, 551, 497 cm−1; HRMS (ESI): calcd for C32H31N3NaO3S: [M + Na]+ 560.1978, found: 560.1973%.
4-Methyl-N-(2-(1-((2-oxopyrrolidin-1-yl)(thiophen-2-yl)methyl)-1H-indol-3-yl)ethyl)benzenesulfonamide (4o). White solid (m.p. 99–100 °C), 12% yield (29.0 mg); 1H NMR (400 MHz, CDCl3) δ 7.89 (s, 1H), 7.80–7.75 (m, 1H), 7.64 (t, J = 6.7 Hz, 2H), 7.51–7.32 (m, 3H), 7.25–7.19 (m, 3H), 7.19–7.10 (m, 1H), 7.09–7.04 (m, 1H), 6.99 (s, 1H), 4.62–4.49 (m, 1H), 3.40 (t, J = 7.0 Hz, 1H), 3.30–3.22 (m, 2H), 2.95–2.85 (m, 2H), 2.74–2.65 (m, 1H), 2.54–2.43 (m, 1H), 2.39 (s, 3H), 2.37–2.27 (m, 1H), 2.10–1.99 (m, 1H), 1.95–1.84 (m, 1H); 13C NMR (75 MHz, CDCl3) δ 177.61, 145.82, 141.09, 139.42, 139.06, 132.12, 132.08, 129.97, 129.47, 129.43, 128.97, 126.83, 125.36, 123.02, 121.32, 114.48, 113.11, 63.43, 45.59, 45.52, 33.16, 28.14, 24.00, 20.44; IR (KBr) νmax: 3389, 2926, 1673, 1596, 1458, 1318, 1156, 1090, 1078, 815, 742, 640, 545, 504 cm−1; HRMS (ESI): calcd for C26H27N3NaO3S2: [M + Na]+ 516.1386, found: 516.1393%.
4-Methyl-N-(2-(2-((2-oxopyrrolidin-1-yl)(thiophen-2-yl)methyl)-1H-indol-3-yl)ethyl)benzenesulfonamide (3o). White solid (m.p. 137–138 °C), 73% yield (179.6 mg); 1H NMR (400 MHz, CDCl3) δ 8.82 (s, 1H), 7.61 (d, J = 7.8 Hz, 2H), 7.46 (d, J = 7.8 Hz, 1H), 7.33 (d, J = 8.0 Hz, 1H), 7.30–7.26 (m, 1H), 7.23–7.16 (m, J = 9.0 Hz, 3H), 7.09 (t, J = 7.3 Hz, 1H), 6.98–6.93 (m, 1H), 6.77 (s, 1H), 6.59 (s, 1H), 4.70 (s, 1H), 3.77–3.69 (m, 1H), 3.47–3.38 (m, 1H), 3.26–3.12 (m, 2H), 3.08–2.93 (m, 2H), 2.47 (t, J = 7.9 Hz, 2H), 2.38 (s, 3H), 2.13–2.01 (m, 2H); 13C NMR (75 MHz, CDCl3) δ 175.78, 143.07, 141.23, 136.79, 135.71, 131.88, 129.53, 127.24, 127.17, 126.98, 126.21, 125.46, 122.73, 119.79, 118.83, 111.52, 110.71, 48.65, 46.25, 43.36, 31.14, 24.61, 21.51, 18.21; IR (KBr) νmax: 3354, 3175, 2935, 2868, 1652, 1597, 1490, 1459, 1425, 1301, 1146, 1095, 933, 812, 748, 721, 672, 574, 542 cm−1; HRMS (ESI): calcd for C26H27N3NaO3S2: [M + Na]+ 516.1386, found: 516.1382%.
N-(2-(1-((2-Oxopyrrolidin-1-yl)(phenyl)methyl)-1H-indol-3-yl)ethyl)acetamide (4p). White solid (m.p. 102–103 °C), 23% yield (42.3 mg); 1H NMR (400 MHz, CDCl3) δ 7.78 (s, 1H), 7.61 (d, J = 7.6 Hz, 1H), 7.48 (d, J = 7.9 Hz, 1H), 7.45–7.38 (m, 3H), 7.24–7.19 (m, 3H), 7.18–7.10 (m, 1H), 6.79 (s, 1H), 5.49 (s, 1H), 3.59–3.44 (m, 3H), 2.96–2.87 (m, 2H), 2.64–2.64 (m, 1H), 2.59–2.49 (m, 1H), 2.47–2.33 (m, 1H), 2.15–2.03 (m, 1H), 1.99–1.87 (m, 4H); 13C NMR (75 MHz, CDCl3) δ 175.59, 170.03, 136.98, 134.95, 128.99, 128.76, 128.19, 126.86, 124.18, 122.71, 120.31, 118.95, 113.09, 110.61, 63.74, 43.35, 39.83, 30.76, 25.28, 23.30, 17.98; IR (KBr) νmax: 3308, 2942, 1696, 1637, 1550, 1459, 1448, 1411, 1278, 1261, 765, 742, 703, 484, 444 cm−1; HRMS (ESI): calcd for C23H25N3NaO2: [M + Na]+ 398.1839, found: 398.1840%.
N-(2-(2-((2-Oxopyrrolidin-1-yl)(phenyl)methyl)-1H-indol-3-yl)ethyl)acetamide (3p). White solid (m.p. 147–148 °C), 30% yield (53.6 mg); 1H NMR (400 MHz, CDCl3) δ 8.75 (s, 1H), 7.64 (d, J = 7.6 Hz, 1H), 7.37–7.27 (m, 4H), 7.23–7.18 (m, 1H), 7.17–7.10 (m, 3H), 6.44 (s, 1H), 5.87 (s, 1H), 3.62–3.42 (m, 4H), 3.07–2.96 (m, 2H), 2.53–2.41 (m, 2H), 2.15–2.06 (m, 2H), 1.76 (s, 3H); 13C NMR (75 MHz, CDCl3) δ 175.69, 170.35, 137.34, 135.76, 132.20, 128.85, 127.82, 127.49, 126.90, 122.61, 119.74, 118.98, 112.27, 111.32, 52.26, 47.14, 40.31, 31.30, 24.26, 23.01, 18.38; IR (KBr) νmax: 3276, 3089, 2940, 1652, 1563, 1492, 1459, 1433, 1285, 1270, 794, 743, 731, 703, 501 cm−1; HRMS (ESI): calcd for C23H25N3NaO2: [M + Na]+ 398.1839, found: 398.1840%.
tert-Butyl(2-(1-((2-oxopyrrolidin-1-yl)(phenyl)methyl)-1H-indol-3-yl)ethyl)carbamate (4q). White solid (m.p. 50–51 °C), 32% yield (69.8 mg); 1H NMR (400 MHz, CDCl3) δ 7.79 (s, 1H), 7.62 (d, J = 7.7 Hz, 1H), 7.49 (d, J = 8.2 Hz, 1H), 7.44–7.37 (m, 3H), 7.23 (d, J = 7.4 Hz, 3H), 7.18 (t, J = 7.5 Hz, 1H), 6.79 (s, 1H), 4.63 (s, 1H), 3.50–3.43 (m, 1H), 3.42–3.35 (m, 2H), 2.90 (t, J = 6.7 Hz, 2H), 2.72–2.62 (m, 1H), 2.58–2.48 (m, 1H), 2.45–2.34 (m, 1H), 2.13–2.04 (m, 1H), 1.97–1.87 (m, 1H), 1.40 (s, 9H); 13C NMR (75 MHz, CDCl3) δ 7.79 (s, 1H), 7.62 (d, J = 7.7 Hz, 1H), 7.49 (d, J = 8.2 Hz, 1H), 7.44–7.37 (m, 3H), 7.23 (d, J = 7.4 Hz, 3H), 7.18 (t, J = 7.5 Hz, 1H), 6.79 (s, 1H), 4.63 (s, 1H), 3.50–3.43 (m, 1H), 3.42–3.35 (m, 2H), 2.90 (t, J = 6.7 Hz, 2H), 2.72–2.62 (m, 1H), 2.58–2.48 (m, 1H), 2.45–2.34 (m, 1H), 2.13–2.04 (m, 1H), 1.97–1.87 (m, 1H), 1.40 (s, 9H); IR (KBr) νmax: 3346, 2925, 1689, 1506, 1460, 1413, 1364, 1265, 1167, 743, 701, 521, 495, 472, 429 cm−1; HRMS (ESI): calcd for C26H31N3NaO3: [M + Na]+ 456.2258, found: 456.2244%.
tert-Butyl(2-(2-((2-oxopyrrolidin-1-yl)(phenyl)methyl)-1H-indol-3-yl)ethyl)carbamate (3q). White solid (m.p. 147–148 °C), 65% yield (142.6 mg); 1H NMR (400 MHz, CDCl3) δ 8.63 (s, 1H), 7.65 (d, J = 7.3 Hz, 1H), 7.37–7.27 (m, 4H), 7.20 (t, J = 7.2 Hz, 1H), 7.16–7.10 (m, 3H), 6.41 (s, 1H), 4.63 (s, 1H), 3.67–3.57 (m, 1H), 3.46–3.34 (m, 3H), 3.07–2.90 (m, 2H), 2.54–2.43 (m, 2H), 2.16–2.06 (m, 2H), 1.37 (s, 9H); 13C NMR (75 MHz, CDCl3) δ 175.56, 155.85, 137.31, 135.64, 132.39, 128.81, 127.68, 127.57, 126.63, 122.55, 119.66, 119.15, 112.35, 111.24, 52.46, 47.38, 41.23, 31.29, 28.35, 24.92, 18.35; IR (KBr) νmax: 3428, 3274, 2965, 2930, 1710, 1665, 1585, 1494, 1455, 1434, 1389, 1365, 1310, 1262, 1165, 1015, 958, 927, 861, 801, 748, 701, 660, 499 cm−1; HRMS (ESI): calcd for C26H31N3NaO3: [M + Na]+ 456.2258, found: 456.2241%.
4-Methyl-N-(2-((1-((2-oxopyrrolidin-1-yl)(phenyl)methyl)-1H-indol-3-yl)methyl)phenyl)benzenesulfonamide (4r). White solid (m.p. 96–97 °C), 14% yield (39.3 mg); 1H NMR (400 MHz, CDCl3) δ 7.81 (s, 1H), 7.50 (d, J = 8.4 Hz, 1H), 7.48–7.38 (m, 3H), 7.35 (d, J = 7.9 Hz, 3H), 7.25–7.20 (m, 4H), 7.18 (d, J = 7.6 Hz, 1H), 7.14–7.07 (m, 4H), 7.04 (t, J = 7.4 Hz, 1H), 6.75 (s, 1H), 6.64 (s, 1H), 3.71 (s, 2H), 3.55–3.47 (m, 1H), 2.81–2.71 (m, 1H), 2.59–2.48 (m, 1H), 2.47–2.38 (m, 1H), 2.35 (s, 3H), 2.15–2.03 (m, 1H), 2.02–1.92 (s, 1H); 13C NMR (75 MHz, CDCl3) δ 175.77, 143.75, 137.52, 136.49, 135.47, 134.61, 131.53, 130.56, 129.57, 129.11, 128.79, 127.76, 126.93, 126.84, 125.62, 124.35, 123.22, 123.18, 120.57, 119.57, 112.60, 110.82, 63.72, 43.38, 30.77, 29.27, 21.54, 18.03; IR (KBr) νmax: 3430, 3059, 2921, 2850, 2364, 1672, 1599, 1492, 1459, 1420, 1333, 1267, 1236, 1159, 1091, 925, 822, 746, 663, 576, 563, 544 cm−1; HRMS (ESI): calcd for C33H31N3NaO3S: [M + Na]+ 572.1978, found: 572.1961%.
4-Methyl-N-(2-((2-((2-oxopyrrolidin-1-yl)(phenyl)methyl)-1H-indol-3-yl)methyl)phenyl)benzenesulfonamide (3r). White solid (m.p. 97–98 °C), 83% yield (227.8 mg); 1H NMR (400 MHz, CDCl3) δ 8.35 (s, 1H), 7.45 (d, J = 7.5 Hz, 2H), 7.28–7.16 (m, 5H), 7.15–7.05 (m, 5H), 7.04–2.97 (m, 4H), 6.96–6.90 (m, 2H), 6.34 (s, 1H), 3.95 (d, J = 16.7 Hz, 1H), 3.76 (d, J = 16.7 Hz, 1H), 3.29–3.21 (m, 1H), 2.88–2.79 (m, 1H), 2.31 (s, 3H), 2.27–2.19 (m, 1H), 1.98–1.79 (m, 2H), 1.67–1.56 (s, 1H); 13C NMR (75 MHz, CDCl3) δ 175.67, 143.46, 137.12, 136.60, 136.11, 135.41, 134.20, 132.55, 129.66, 129.55, 128.89, 128.06, 127.81, 127.23, 126.95, 126.65, 126.57, 125.93, 122.66, 120.03, 118.90, 112.51, 111.38, 51.76, 46.23, 30.81, 25.43, 21.58, 17.82; IR (KBr) νmax: 3361, 3060, 2924, 2853, 1664, 1599, 1493, 1458, 1419, 1332, 1287, 1159k, 1091, 922, 815, 745, 662, 561, 496 cm−1; HRMS (ESI): calcd for C33H31N3NaO3S: [M + Na]+ 572.1978, found: 572.1966%.
Acknowledgements
We gratefully acknowledge the Natural Science Foundation of China (no. 21172162), the Young National Natural Science Foundation of China (no. 21202111), the Young Natural Science Foundation of Jiangsu Province (BK2012174), PAPD, Key Laboratory of Organic Synthesis of Jiangsu Province (KJS1211), and Soochow University for financial support.
Notes and references
-
(a) D. G. Barceloux, Tryptamine Designer Drugs, in Medical Toxicology of Drug Abuse, John Wiley & Sons, Inc., Hoboken, 2012, p. 193 Search PubMed;
(b) A. J. Kochanowska-Karamyan and M. T. Hamann, Chem. Rev., 2010, 110, 4489 CrossRef CAS PubMed.
-
(a) M. E. Speeter and W. C. Anthony, J. Am. Chem. Soc., 1954, 76, 6208 CrossRef CAS;
(b) A. M. Schmidt and P. Eilbracht, J. Org. Chem., 2005, 70, 5528 CrossRef CAS PubMed.
-
(a) V. G. Nenajdenko, E. P. Zakurdaev, E. V. Prusov and E. S. Balenkova, Tetrahedron, 2004, 60, 11719 CrossRef CAS PubMed;
(b) K. J. Hwang and T. S. Lee, Synth. Commun., 1999, 29, 2099 CrossRef CAS.
- M. Righi, F. Topi, S. Bartolucci, A. Bedini, G. Piersanti and G. Spadoni, J. Org. Chem., 2012, 77, 6351 CrossRef CAS PubMed.
-
(a) J. S. Kim, A. Padnya, M. Weltzin, W. Edmonds, K. Schulte and R. A. Glennon, Bioorg. Med. Chem. Lett., 2007, 17, 4855 CrossRef CAS PubMed;
(b) N. German, J. S. Kim, A. Jain, M. Dukat, A. Pandya, Y. Ma, M. Weltzin, M. K. Schulte and R. A. Glennon, J. Med. Chem., 2011, 54, 7259 CrossRef CAS PubMed.
-
(a) R. H. Snell, M. J. Durbin, R. L. Woodward and M. C. Willis, Chem.–Eur. J., 2012, 18, 16754 CrossRef CAS PubMed;
(b) T. Newhouse, C. A. Lewis, K. J. Eastman and P. S. Baran, J. Am. Chem. Soc., 2010, 132, 7119–7137 CrossRef CAS PubMed.
-
(a) R. L. Noble, C. T. Beer and J. H. Cutts, Ann. N. Y. Acad. Sci., 1958, 76, 882 CrossRef CAS;
(b) G. H. Svoboda, N. Neuss and M. J. Gonnan, J. Am. Pharm. Assoc., Sci. Ed., 1959, 48, 659 CrossRef CAS.
-
(a) E. K. Rowinsky and R. C. Donehower, Cancer Chemotherapy and Biotherapy, ed. B. A. Chbner and D. L. Longo, Lippincott-Raven Publishers, Philadelphia, PA, 1996, p. 263 Search PubMed;
(b) C. Schneider, Angew. Chem., Int. Ed., 2002, 41, 4217 CrossRef CAS;
(c) T. Miyazaki, S. Yokoshima, S. Simizu, H. Osada, H. Tokuyama and T. Fukuyama, Org. Lett., 2007, 9, 4737 CrossRef CAS PubMed.
- H.-Y. Xu, X.-P. Xu, S.-Y. Wang and S.-J. Ji, Eur. J. Org. Chem., 2012, 5440 CrossRef CAS.
-
(a) R. C. Larock and E. K. Yum, J. Am. Chem. Soc., 1991, 113, 6689 CrossRef CAS;
(b) R. C. Larock, E. K. Yum and M. D. Refvik, J. Org. Chem., 1998, 63, 7652 CrossRef CAS;
(c) Y. Dong and C. A. Busacca, J. Org. Chem., 1997, 62, 6464 CrossRef CAS;
(d) P. Linnepe, A. M. Schmidt and P. Eilbracht, Org. Biomol. Chem., 2006, 4, 302 RSC.
- M. E. Kieffer, L. M. Repka and S. E. Reisman, J. Am. Chem. Soc., 2012, 134, 5131 CrossRef CAS PubMed.
- G. Buchi and C. P. Mak, J. Org. Chem., 1977, 42, 1784 CrossRef.
- K. C. Nicolaou, A. Krasovskiy, U. Majumder, V. E. Trepanier and D. Y.-K. Chen, J. Am. Chem. Soc., 2009, 131, 3690 CrossRef CAS PubMed.
-
(a) U. Hary, U. Roettig and M. Paal, Tetrahedron Lett., 2001, 42, 5187 CrossRef CAS;
(b) F. Hadjaz, S. Yous, N. Lebegue, P. Berthelot and P. Carato, Tetrahedron, 2008, 64, 10004 CrossRef CAS PubMed.
- H.-Y. Xu, S.-Y. Wang, R. Jiang, X.-P. Xu, X.-Q. Chu and S.-J. Ji, Tetrahedron, 2012, 68, 8340 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 947549 and 947550. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c3ra44196b |
|
| This journal is © The Royal Society of Chemistry 2014 |
Click here to see how this site uses Cookies. View our privacy policy here. ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5).
5).![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4a
4a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8
8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 34
34![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 24
24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 32
32![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 43
43![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 18
18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30
30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 52
52![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 23
23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 62
62![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4a
4a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8
8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4
4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 33
33![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6
6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 molar ratio of 1a and 2a in the presence of 15 mol% In(OTf)3 in CH2Cl2 for 10 hours, which could furnish 3a in 74% LC-yield (73% isolated yield) and 4a in 14% LC-yield, respectively (Table 4, entry 2).
1.5 molar ratio of 1a and 2a in the presence of 15 mol% In(OTf)3 in CH2Cl2 for 10 hours, which could furnish 3a in 74% LC-yield (73% isolated yield) and 4a in 14% LC-yield, respectively (Table 4, entry 2).![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4a
4a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8
8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 46
46![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25
25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12
12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 13
13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 13
13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 17
17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 16
16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2a
2a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4a
4a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12
12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5
1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 16
16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 26
26![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.5
2.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 35
35![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 chemoselectivity (Table 5, entries 1–5). The substrates 2 bearing Ar with strong electron-withdrawing groups, such as nitro and cyano groups, could proceed well to give the desired products in excellent yields with up to 85
5 chemoselectivity (Table 5, entries 1–5). The substrates 2 bearing Ar with strong electron-withdrawing groups, such as nitro and cyano groups, could proceed well to give the desired products in excellent yields with up to 85![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15 chemoselectivity (Table 5, entries 11 and 12). Some other substrates 2 bearing aryls, such as naphthalene-2-yl and thiophen-2-yl, were demonstrated to be good candidates for this reaction, giving high yields (Table 5, entries 13 and 14). As shown in Table 5, the substrates 2 bearing Ar with the same group at different positions gave similar results, reflecting that the steric effect has little influence (Table 5, entries 1–5, 7–10). As shown in Table 5, 2-substituted tryptamines were formed in a higher ratio compared to N-substituted tryptamines due to the steric congestion caused during the attack from different positions of the indole to the bulky iminium ion. The absolute configurations of 3k and 4f have been determined by X-ray diffraction (Scheme 4).
15 chemoselectivity (Table 5, entries 11 and 12). Some other substrates 2 bearing aryls, such as naphthalene-2-yl and thiophen-2-yl, were demonstrated to be good candidates for this reaction, giving high yields (Table 5, entries 13 and 14). As shown in Table 5, the substrates 2 bearing Ar with the same group at different positions gave similar results, reflecting that the steric effect has little influence (Table 5, entries 1–5, 7–10). As shown in Table 5, 2-substituted tryptamines were formed in a higher ratio compared to N-substituted tryptamines due to the steric congestion caused during the attack from different positions of the indole to the bulky iminium ion. The absolute configurations of 3k and 4f have been determined by X-ray diffraction (Scheme 4).![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4
4
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4b = 86
4b = 86![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 14
14
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4c = 78
4c = 78![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 22
22
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4d = 95
4d = 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5
5
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4e = 82
4e = 82![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 18
18
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4f = 68
4f = 68![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 32
32
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4g = 79
4g = 79![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 21
21
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4h = 85
4h = 85![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15
15
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4i = 82
4i = 82![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 18
18
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4j = 77
4j = 77![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 23
23
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4k = 88
4k = 88![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12
12
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4l = 72
4l = 72![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 28
28
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4m = 85
4m = 85![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15
15
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4n = 82
4n = 82![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 18
18
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4o = 86
4o = 86![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 14
14
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4h = 89
4h = 89![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11
11
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4m = 89
4m = 89![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11
11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 14). Unfortunately, it was found that no desired product was formed when tryptamine was used in the reaction, under identical reaction conditions.
14). Unfortunately, it was found that no desired product was formed when tryptamine was used in the reaction, under identical reaction conditions.