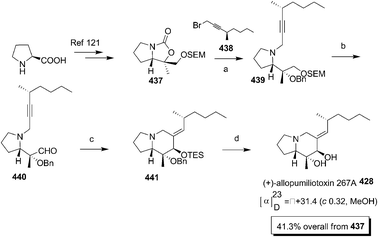
Recent advances in the synthesis of naturally occurring pyrrolidines, pyrrolizidines and indolizidine alkaloids using proline as a unique chiral synthon
Chinmay Bhat
and
Santosh G. Tilve
*
Department of Chemistry, Goa University, Taleigao-Plateau, Goa 403 206, India. E-mail: stilve@unigoa.ac.in; santoshtilve@yahoo.com; Fax: +91-0832-2452889; Tel: +91-0832-6519317 Tel: +91-0832-2452886
First published on 22nd October 2013
Abstract
The present article describes the synthesis of a wide spectrum of natural products of the class pyrrolidines, pyrrolizidines and indolizidines using proline as a viable synthetic precursor. The review emphasizes the versatility of the basic unit of proline as a useful chiral synthon confined for the synthesis of only natural products of the above mentioned families. The vast coverage of the synthesis of these natural products is presented for a period from 1990 onwards. The synthesis of all ranges of alkaloids from simple to complex molecules is presented under the groups of alkaloids.
1. Introduction
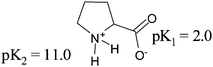
Over the last few decades, asymmetric synthesis of natural products has gained major importance from an industrial and academic relevance.1 Asymmetric synthesis mainly involves; carrying out the reaction with the integrity of the chiral centre viz. “chiral pool” strategy, introducing new chiral centres by chiral induction methods, use of chiral auxiliaries and organocatalysis. It is always difficult to carry out selective transformation of the molecules to generate new chiral centres. Although the use of organocatalysis is soaring nowadays, selectivity towards the substrates, efficacy of the systems and cost of organocatalysts make this cumbersome from an economic point of view. On the other hand, the “chiral pool” approach, being an effectual paradigm, plays a very prominent role from synthetic relevance2 as the starting materials are easily carved from readily available materials like amino acids, carbohydrates, terpenes and organic acids. A vast number of natural products are derived from different amino acids3 due to the availability of the functional groups suitable for various transformations to effect the required modifications and constitute new appendages. As a consequence, several amino acids have prompted synthetic chemists to contemplate designing various synthetic routes to embody different natural products and their structural entities.Proline is a bifunctional, non-essential amino acid prevalent in various natural and synthetic bioactive molecules. It is the only cyclic amino acid, synthesized in our body. Despite being structurally an imino acid, it is popularly called an amino acid. L-Proline, is abundant in nature, cheaply available commercially, and finds application in various pharmacological and biotechnological applications due to its osmoprotectant behaviour. Proline is a widely distributed osmolyte found to accumulate in several environmentally stressed plants as well as microorganisms.4 It is also used as a nitrogen source during fermentation of grape musts for the production of wine. It is the only amino acid which attains cis configuration in peptides unlike other amino acids which normally exist in trans form. Due to this unique behaviour, it assisted the detail study of protein folding and cis–trans isomerisation. L-Proline is one of the two amino acids which disobeys the popular “Ramachandran plot”, the other being glycine.
The unique structure of proline, having both carboxylic and imino groups (Fig. 1) prevails as a versatile organocatalyst through enamine and iminium ion mechanism. Consequently, over the last few decades various proline derived organocatalysts with a multitude of embellishments have been articulated by appropriate transformation of its functional groups and efficiently applied for enantioselective and diastereoselective reactions.5 Proline enunciates its effect as a profoundly versatile ligand by complexing with the various metals for the synthetic transformation of organic molecules.6 The availability of the five member ring with a stereogenic nitrogen centre and the two functional groups (Fig. 2) in combination inflict the transformation of the molecule into a myriad of naturally occurring pyrrolidines, pyrrolizidines, piperidines, quinolizidines, indolizidines and macrocycles ranging from simple to complex molecules. Proline being a natural product; conversion of it to other natural products reflects the competency in adaptation of one natural product into another. The biosynthesis of L-proline is derived from L-glutamate and L-ornithine (Scheme 1).4 Synthetic proline was reported by Willstätter in 1900 using sodium salt of diethyl malonate and 1,3-dibromopropane.
The present review delineates the use of (R) and (S) proline for the synthesis of the aforementioned types of alkaloids. The comprehensive coverage of the syntheses of these alkaloids has been done (since 1990 to 2013) using proline as a starting material or the major synthetic precursor. The synthetic applications of numerous C-substituted proline units, namely hydroxyl-prolines, also found as useful precursors for the synthesis of these alkaloids is not within the scope of this review. The synthesis of several macro cyclic compounds, generally polypeptides, was also ventured using proline as a precursor, and is also not included in this review. The review focuses directly only on the application of proline transforming to natural products and not on the isolation and biological assays of the synthesized natural product.
2. Synthesis of pyrrolidine alkaloids
2.1. Introduction
Pyrrolidine alkaloids bearing five member N-heterocycles, are enormously ubiquitous in various natural7 and unnatural components.8 There are about 80 pyrrolidine alkaloids known with hygrine being the simplest. These alkaloids are mainly extracted from the plants of families Colanaceae, Convolvunaceae and Erythroxylaceae. These classes of alkaloids constitute a part of the organocatalysts9 and building blocks in organic synthesis.10 They are endowed with a host of biological activities and pharmacological behaviours. The difficulties in the isolation and purification of these alkaloids and the global scarcity has imposed on synthetic chemists the need to contemplate the design of novel synthetic schemes. Proline, being one of the simple pyrrolidine alkaloids, has been found to be a viable precursor for the synthesis of these alkaloids through systematic transformation of the functional groups. The simplest pryrrolidine alkaloid, hygrine, acts as a biogenetic precursor for tropane alkaloids.2.2. Tropane and sedum alkaloids
These classes of alkaloids are mainly 2-substituted pyrrolidine and piperidine members with different functional groups on the side chain and have attracted immense interest from synthetic chemists due to their intriguing pharmacological activities and hallucinogenic characteristics.11 These alkaloids were mainly isolated from the plants Schizanthus hookeri, Carallia brachiata and Erythroxylon coca. Some of the representative members of five member alkaloids include (+)-hygrine 1, (+)-hygroline 2 and (+)-pseudohygroline 3, etc.Shono et al. have synthesized (+)-hygroline 2 and (+)-pseudohygroline 3 starting from proline using anodic oxidation as a key step (Scheme 2).12 The L-proline was efficiently converted to 4 as a mixture of diastereomers 4a and 4b according to previously reported methods.13 The mixture was separated on column chromatography. The further reaction of either 4a or 4b with isopropenyl acetate in the presence of TiCl4 resulted in an enantiomeric mixture of 5a and 5b which was as such hydrolysed using alkaline solution and electrochemically oxidised in MeOH to give a mixture of 6a and 6b. Further reduction of carbamate with LAH gave a mixture of isomers of hygroline 2a and 2b and pseudohygroline 3a and 3b which were separated with optical purity of 42.60% and 45.62% respectively using preparative GLC.
 | ||
| Scheme 2 Reagents and conditions: (a) prenyl acetate, TiCl4, 85%; (b) (i) KOH, (ii) −2e, CH3OH, NaOMe (anodic oxidation), 52%; (c) LAH, THF, refux, 81%. | ||
Arévalo-García and Colmenares synthesized the tropane pyrrolidine alkaloid (+)-hygrine 1, mainly found in coca leaves, in six steps (Scheme 3) using (R)-proline derived ester 7 as a chiral precursor.14 N-Methylated proline ester 7 was reduced to aldehyde 8 using DIBAL which was further homologated to 9 by reaction with PPh3![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHOCH3 followed by acid hydrolysis. The Grignard reaction on 9 with MeMgBr and subsequent oxidation with DMP gave (+)-hygrine 1.
CHOCH3 followed by acid hydrolysis. The Grignard reaction on 9 with MeMgBr and subsequent oxidation with DMP gave (+)-hygrine 1.
 | ||
| Scheme 3 Reagents and conditions: (a) DIBAL, DCM, −30 °C; (b) PPh3CH2OCH3Br, KOtBu, THF, 10% HCl–THF; (c) (i) MeMgBr, THF; (ii) DMP, DCM. | ||
Our research group has also approached the synthesis of the pyrrolidine alkaloids using proline as a starting material.15 (−)-Hygrine 10 and (−)-norhygrine 11 were synthesized using regioselective Wacker oxidation as a key step (Scheme 4). The synthesis started with commercially available L-proline, converted to N-Cbz-prolinal 12a. The aldehyde 12a on Wittig reaction with ethylidinephosphorane afforded cis olefin 13. The Wacker oxidation of the non-terminal double bond of 13, performed using PdCl2–CuCl in O2 took place regioselectively at the carbon atom further away from the ring due to the bulky Cbz group, delivering the keto product 14a. The keto group of 14a was converted to its acetal form to give 15 before reducing the N-Cbz group to N-methyl using LAH in refluxing THF to give 16. The amine 16 thus formed was treated with HCl to afford the natural product (−)-hygrine 10 as the hydrochloride salt. Incidentally the first synthesis of (−)-norhygrine 11 which is usually found along with hygrine in nature, was carried out by selective deprotection of the Cbz group of 14a by hydrogenation over Pd/C.
In a continuation of our interest using proline as a synthetic predecessor, we recently reported the total synthesis of tropane and sedum alkaloids namely (−)-hygrine 10, (−)-norhygrine 11, (−)-hygroline 17 and (−)-pseudohygroline 18 through Henry and Nef reactions (Scheme 5).16 The aldehydes 12a–c prepared from proline were subjected to Henry reaction using excess nitroethane and a catalytic amount of KOH in methanol to give diastereomeric mixtures of nitro aldol products which without separation on subsequent mesylation followed by treatment with Et3N, afforded nitro olefins 19a–c. The pivotal Nef reaction was successfully performed using NaBH4–MeOH–H2O2 and K2CO3 without racemisation to afford 14a–c. The syntheses of aforementioned alkaloids were straightforwardly carried out by reduction of carbonyl and suitable deprotection and reduction of the protecting groups of 14a–c. (−)-Norhygrine 11 was prepared by hydrogenolysis of the benzylcarbamate group of 14a over Pd/C while synthesis of (−)-hygrine 10 was achieved starting from 14c by LAH reduction followed by DMP oxidation. (−)-Hygroline 17 and (−)-pseudohygroline 18 were synthesized directly by reducing the ethyl carbamate group of 20 and 21 respectively using LAH.
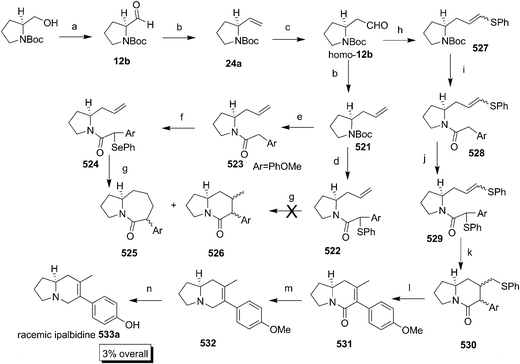
Another important pyrrolidine alkaloid (−)-dihydrocuscohygrine 22a was isolated from Erythroxylon coca in 1981 by Turner.17 Recently Yerri et al. synthesized (−)-deoxocuscohygrine 22b using proline as a starting material employing cross metathesis as a key step (Scheme 6).18 The commercially available N-Boc-proline ester 23 on reduction followed by Swern oxidation and Wittig olefination afforded 24a. The alkene 24a on hydroboration followed by subsequent oxidation and Wittig reaction produced the homologated alkene 25, a key intermediate for metathesis. The cross metathesis of 25 and 24a using Grubbs II catalyst resulted in the mixture of homodimers 26 and 27 along with an inseparable cis and trans mixture of required 28 in less yield. It was envisioned that the compound 24a showed sluggish behaviour towards metathesis due to the presence of the bulky Boc group nearer to the olefin group and also the formation of the homodimer 26 was favoured due to its less reversibility. The formation of dimers was then minimised by reacting excess 25 (1.6 equiv.) with 24a, furnishing the required product 28 in 69% yield along with dimer 26 (55% with respect to 27). The product 28 was then separated and subjected to hydrogenation over Pd/C to give 29, followed by LAH reduction to furnish (−)-deoxocuscohygrine 22b.
2.3. Dolastatin: synthesis of dolaproine unit
Dolastatin 10 is a marine natural product consisting of 8 chiral centres, isolated in 1984 by Petit et al. from the sea hare Dolabella auricularia.19 After the elucidation of the structure in 1987, the first synthesis was reported by the same group in 1989.20 The alkaloid has shown a remarkable antineoplastic activity and is under clinical trial for anticancer characteristics.21 Dolastatin 10 is comprised of the 3-chiral centred β-methoxy-γ-amino acid, dolaproine (Dap) 30. Most of the available reports approached the synthesis of dolastatin 10 through the synthesis of Dap 30 unit which can conveniently be accessed from proline.In continuation of isolation research on dolastatin 10, Petit et al. have synthesized the 4 isomers of Boc-Dap 31 unit through aldol condensation (Scheme 7).22 The compound Boc-prolinal 12b on aldol reaction with an enolate of chiral ester 32 using a strong base LDA in combination with MgBr2 resulted in a mixture of diastereomers 33 separable by column chromatography. The compound 33 on treatment with (CH3)3OBF4 afforded the methoxy compound 34 which on hydrogenolysis produced Boc-Dap isomers 31. The configuration of the major isomer 31a was confirmed by converting 33a to the lactam 36 (Scheme 8) through ester 35 and systematic NMR studies. However the required isomer for the synthesis of dolaproine 30 was 33b, found to be formed in low yield.
 | ||
| Scheme 7 Reagents and conditions: (a) iPr2NLi, MgBr2·Et2O; (b) (CH3)3OBF4, proton sponge; (c) H2, 10% Pd/C. | ||
 | ||
| Scheme 8 Reagents and conditions: (a) (i) H2–5% Pd/C, (ii) CH2N2; (67% for two steps); (b) (i) TFA, (ii) K2CO3 (71% for two steps). | ||
Petit et al. further improved the chiral synthesis of Dap 30, enantioselectively and diastereoselectively through dibutyl boron triflate [(Bu)2BOTf] mediated aldol condensation (Evans method) (Scheme 9).23 The Boc-prolinal 12b was treated with chiral oxazolidinone 37 in the presence of (Bu)2BOTf and Et3N to furnish the compound 38 as a single diastereomer. The synthesis of the Boc-Dap 31b was then achieved through two converging routes, either by methylation followed by hydrolysis of the chiral auxiliary (38–39–31b) or by hydrolysis of the chiral auxiliary followed by methylation (38–40–31b). In a similar way Hamada and co-workers approached the synthesis of dolastatin 10 by synthesizing using the intermediate N-Boc-Dap 31b.24 The reaction of Boc prolinal 12b with chiral auxiliary 41 gave a separable mixture of isomers 42a and 42b. The compound 42a was then converted to 31b by hydrolysis of the chiral auxiliary followed by methylation. The configuration of the isomers was confirmed by converting them to known compounds and comparing with those reported. An interesting phenomenon was observed that only the cis isomer 42a was formed with complete diastereoselection using slight excess Et3N while the major anti product 42b was formed when (Bu)2BOTf was used in excess. The required cis product 42a was then converted to the dolaproine unit 31b by hydrolysing the chiral auxiliary using LiOH and H2O2 and followed by methylation of –OH using MeI.
 | ||
| Scheme 9 Reagents and conditions: (a) (Bu)2OTf, Et3N, −75 °C to rt; (b) (CH)3O+BF4−, proton sponge, 0 °C to rt, 46 h; (c) LiOH–H2O2, Na2SO3, 3 °C to rt, 16 h; (d) NaH, MeI, 0 °C, 48 h. | ||
A few years later, Petit and Grealish once again succeeded in getting the required isomeric intermediate of Dap 30 in major amounts by carrying out stereoselective Reformatsky reaction assisted by a cobalt–phosphine complex (Scheme 10).25 The reaction of the Boc protected prolinal 12b with bromo amide 43 diastereoselectively led to 44. The free –OH in 44 was methylated using trimethyloxonium tetrafluoroborate BF4O(CH3)3 to give 45. The chiral auxiliary unit was then hydrolysed using LiOH and H2O2 to furnish Boc-Dap 31b.
 | ||
| Scheme 10 Reagents and conditions: (a) Co[P(PPh3)4], THF, 0 °C, 70%; (b) BF4O(CH3)3, proton sponge, 4 Å MS, DCM, 86%; (c) LiOH, H2O2, 94%. | ||
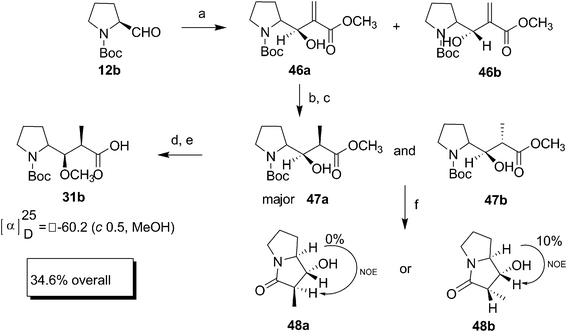
Almeida and Coelho have synthesized Boc-Dap 31b by coupling N-Boc-prolinal 12b and methyl acrylate through Baylis–Hillman reaction (Scheme 11)26 in four steps with an overall yield of 27%. The Baylis–Hillman reaction was performed using ultrasound sonication, which without racemisation led to a mixture of diastereomers 46, separable on column chromatography. The major isomer formed was predicted to be 46a based on a Felkin–Ahn open-chain model which on hydrogenation afforded a mixture of isomers 47a and 47b in the ratio of 87![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 17. To determine the configuration of the chiral centres, the separated diastereomers of 47 were subjected to cylclisation to give lactam 48a and 48b, whose NOE study confirmed the formation of the required isomer in major amount. The further confirmation of the structure was done by converting 47a to well known compound Boc-Dap 31b by successful methylation of the hydroxyl group and hydrolysis of the ester group, which completed the formal synthesis of dolastatin 10.
17. To determine the configuration of the chiral centres, the separated diastereomers of 47 were subjected to cylclisation to give lactam 48a and 48b, whose NOE study confirmed the formation of the required isomer in major amount. The further confirmation of the structure was done by converting 47a to well known compound Boc-Dap 31b by successful methylation of the hydroxyl group and hydrolysis of the ester group, which completed the formal synthesis of dolastatin 10.
 | ||
| Scheme 12 Reagents and conditions: (a) (i) ImCOIm, THF, 0 °C–rt, 3 h; (ii) 49, THF, Et2O, −10 °C–rt, 4 days, (82% for two steps); (b) HCl gas, EtOH, 0 °C, 2 h. | ||
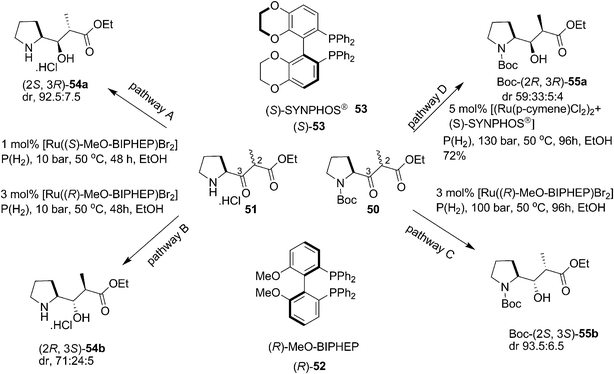
Genet and co-workers approached the synthesis of dolastatin 10 via dynamic kinetic resolution (DKR) of 50 and 51 (Scheme 12) by performing an efficient catalytic asymmetric hydrogenation using Ru complexed with chiral ligands (Scheme 13).27 The great discovery of manipulation of catalyst, temperature, solvent conditions and nature of protecting groups on N atom were studied for DKR of different amino substrates during the synthetic manoeuvring of γ-amino acids. For the synthesis of Boc-dap isomers, the Boc-protected and deprotected proline units showed remarkable changes in diastereoselectivity. Anti selectivity at the 2nd and 3rd positions was observed with unprotected proline unit 51 with Ru complexed ligands (S)-52 and (R)-52 to give 54a and 54b. The best selectivity was observed in pathway A. Surprising failure to achieve high cis selectivity turned attention to perform the reaction with a Boc protected unit 50. The moderate cis selectivity was then achieved using (S)-53 and (R)-52 giving 55a and 55b respectively as major isomers by performing the hydrogenation under very high pressure and elongated reaction period. The product 55a synthesized this way was efficiently transformed to Boc-dap 31 (Scheme 14) after separating it from minor isomers which on further synthetic conversion was eventually converted to dolastatin 10.
 | ||
| Scheme 14 Reagents and conditions: (a) LHMDS, HMPA, THF, −78 °C, 25 min, then MeOTf, −20 °C, 15 min, 45%; (b) LiOH, EtOH–H2O, overnight, 59%. | ||
Cella et al. studied the diastereoselective addition of crotyl trifluoroborate salts on α-amino aldehydes and successfully synthesized Boc-dap 31b in the presence of PTC (Bu4NI) (Scheme 15).28 The configuration of 57a was confirmed by converting it to 58 and measuring the proton coupling constant of vicinal protons. The compound 57a was methylated and further converted to acid 31b using RuO2 which constituted the formal synthesis of dolastatin 10.
 | ||
| Scheme 15 Reagents and conditions: (a) n-Bu4NI (10 mol%), DCM–H2O, 89%; (b) NaH, MeI, DMF, 76%; (c) RuO2, CH3CN–H2O–CHCl3, 75%; (d) NaH, THF, 90%. | ||
The work done by Poncet and co-workers involves the addition of crotyl boronate 61 to Boc-prolinal 12b giving all the possible four isomers 57 with the requisite 57a as a major isomer.29 The configuration of each isomer was determined by different experimentation. Compound 57a on methylation and subsequent oxidation using RuO2 afforded Boc-dap 31b unit (Scheme 16).
 | ||
Scheme 16 Reagents and conditions: (a) MeNHOMe![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) HCl, BOP, DIEA, DCM, 82%; (b) LAH, THF, 89%; (c) 61, THF, 64%; (d) NaH, MeI, DMF, 90%; (e) RuO4, CCl4, CH3CN, H2O, 81%. HCl, BOP, DIEA, DCM, 82%; (b) LAH, THF, 89%; (c) 61, THF, 64%; (d) NaH, MeI, DMF, 90%; (e) RuO4, CCl4, CH3CN, H2O, 81%. | ||
A notable aldol condensation carried out by Koga and co-workers of boron enolate of thiophenyl propionate 62 with Boc prolinal 12b afforded 63 as a major isomer along with other minor isomers (Scheme 17).30 The compound 63 was then dethionated and esterified to give 55a which on subsequent methylation and ester hydrolysis gave Boc-dap 31b. The configuration of 56 was confirmed by preparing it from the known ester 64.
2.4. Miscellaneous examples
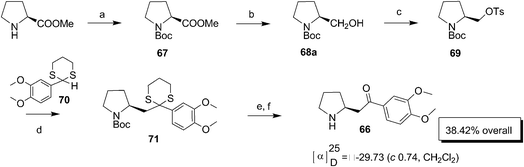
Jones and Woo confirmed the configuration of (−)-ruspolinone 66 as S by synthesizing it starting from S-proline methyl ester (Scheme 18).31 The synthesis suggested that racemisation must have occurred during its isolation from the plant Ruspolia hypercrateriformis.32 The NH of L-proline methyl ester on Boc protection afforded 67. The ester group was selectively reduced to alcohol 68a by LAH at a lower temperature and then tosylated to give 69. The alkylation of anion of the dithiane protected 3,4-dimethoxybenzaldehyde 70 by compound 69 gave 71 in good yield. Further the removal of the dithiane group using NCS and AgNO3 followed by deprotection of the Boc moiety using TFA afforded (−)-ruspolinone 66 as a pale yellow solid.Jerrold Meinwald's group synthesized the defensive alkaloid 2-(12′-aminotridecyl)-pyrrolidine 80 (Scheme 19)33 isolated from the Mexican bean beetle, Epilachna varivestis.34 The Cbz-prolinol 72 on oxidation followed by Wittig reaction with phosphorane obtained from bromo compound 73 gave alkene 74 (E![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Z/3
Z/3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). The acetal group was deprotected to give aldehyde 75 which on Grignard reaction with CH3MgI gave an inseparable diastereomeric mixture of alcohol 76. The alcoholic group of the mixture 76 was tosylated and reacted with NaN3 to afford 78 which on reduction with H2–Pd afforded an inseparable mixture of 79. The separation of the isomers 79 was achieved by making derivatives with additional chiral group attachment and further detail spectroscopic study revealed the structure of the naturally occurring compound as 80 with the configuration (2S,12′R).
1). The acetal group was deprotected to give aldehyde 75 which on Grignard reaction with CH3MgI gave an inseparable diastereomeric mixture of alcohol 76. The alcoholic group of the mixture 76 was tosylated and reacted with NaN3 to afford 78 which on reduction with H2–Pd afforded an inseparable mixture of 79. The separation of the isomers 79 was achieved by making derivatives with additional chiral group attachment and further detail spectroscopic study revealed the structure of the naturally occurring compound as 80 with the configuration (2S,12′R).
Enders and co-workers encompassed the synthesis of 80 using their SAMP–hydrazone methodology35 starting from (R)-proline (Scheme 20).36 The Wittig product 84 prepared through classical synthetic steps was subjected to deprotection of the acetal followed by the trapping of the resultant aldehyde with (S)-1-amino-2-(methoxymethyl) pyrrolidine (SAMP) affording hydrazone 85. The addition of methyl lithium across the nitrogen double bond took place highly diastereoselectively giving exclusively 86. The further reduction of the double bond, benzyl deprotection and cleavage of the N–N bond afforded the natural product 80 in 35% overall yield and with high optical purity.
Blanco et al. performed studies on the configuration of the Pandanus alkaloids by attempting the synthesis of pandamarilactonines 87 (Scheme 21)37 from L-proline. The requisite precursor 88 was prepared from proline according to the literature reports.38 The alkene 88 was oxidised to oxiranes 89a and 89b (1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) using MCPBA and were separated on column chromatography. The major erythro isomer 89a was converted to erythro butenolide 90a along with the formation of threo 90b by reacting with the dianion of 2-phenylselenopropionic acid followed by lactonization and oxidation of the selenide group with consequent elimination. The selective deprotection of the carbamate group of enantiopure 90a using TMSI in CH3CN resulted in concomitant epimerization and racemisation to furnish two norpandamarilactonines 91a and 91b, each of them with a very low optical activity, separable by column chromatography. Further the 1
1) using MCPBA and were separated on column chromatography. The major erythro isomer 89a was converted to erythro butenolide 90a along with the formation of threo 90b by reacting with the dianion of 2-phenylselenopropionic acid followed by lactonization and oxidation of the selenide group with consequent elimination. The selective deprotection of the carbamate group of enantiopure 90a using TMSI in CH3CN resulted in concomitant epimerization and racemisation to furnish two norpandamarilactonines 91a and 91b, each of them with a very low optical activity, separable by column chromatography. Further the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of 91a and 91b was treated with mesylate 92a in pyridine resulting in the separable mixture of 87a and 87b but with complete racemisation.
1 mixture of 91a and 91b was treated with mesylate 92a in pyridine resulting in the separable mixture of 87a and 87b but with complete racemisation.
Takayama et al. achieved the synthesis of enantiomerically pure pandamarilactonine A 87c (Scheme 22).39 The synthesis commenced with Zn metallated Reformatsky reaction on Cbz protected prolinal 12a which led to two diastereomers threo 93a and erythro 93b in the ratio of 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The erythro 93b isomer with unwanted stereochemistry was converted to requisite threo 94 either by converting the ester group of erythro 93b to acid 93c followed by intramolecular Mitsunobu reaction or by oxidising the secondary alcoholic group of erythro 93b to ketone 95 using DMP followed by reduction and cyclisation. The exo to endo isomerisation of the double bond of 94 was performed using Et3SiH (5 mol%) and tris(triphenylphosphine) rhodium chloride (10 mol%) in refluxing toluene giving α-methyl butenolide 96. The relative stereochemistry was established using X-ray analysis. The Cbz group was selectively removed using TMSI in CH3CN at −15 °C to give 97 maintaining the integrity of the chiral centres. The total synthesis was then achieved by coupling the amine 97 with iodo compound 92b to afford pure pandamarilactonine A 87c after purification. The authors speculated the reasons for the racemisation during isolation due to acidic or basic conditions and in nature it is not observed due to the participation of enzymes.
1. The erythro 93b isomer with unwanted stereochemistry was converted to requisite threo 94 either by converting the ester group of erythro 93b to acid 93c followed by intramolecular Mitsunobu reaction or by oxidising the secondary alcoholic group of erythro 93b to ketone 95 using DMP followed by reduction and cyclisation. The exo to endo isomerisation of the double bond of 94 was performed using Et3SiH (5 mol%) and tris(triphenylphosphine) rhodium chloride (10 mol%) in refluxing toluene giving α-methyl butenolide 96. The relative stereochemistry was established using X-ray analysis. The Cbz group was selectively removed using TMSI in CH3CN at −15 °C to give 97 maintaining the integrity of the chiral centres. The total synthesis was then achieved by coupling the amine 97 with iodo compound 92b to afford pure pandamarilactonine A 87c after purification. The authors speculated the reasons for the racemisation during isolation due to acidic or basic conditions and in nature it is not observed due to the participation of enzymes.
Takayama and co-workers isolated another new alkaloid pandamarilactonine-H 98a from the roots of Pandanus amaryllifolius. The systematic structure elucidation was done by different spectroscopic techniques and first total synthesis of its enantiomer 98b starting from D-proline (Scheme 23).41 The synthesis initiated with Cbz protected D-proline converted to anhydride 99 using ethylchloroformate and Et3N. The anhydride 99 on Arndt–Eistert reaction using trimethylsilyldiazomethane in CH3CN afforded α-diazoketone 100 which was subjected to homologation via Wolf rearrangement using silver benzoate to afford 101. The Cbz group hydrogenolysed giving 102 and further, NH was alkylated with 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 (Z/E) mixtures of iodo compound 103 to afford a mixture of diastereomers 98b and 98c separable on column purification. The detail spectroscopic analysis unambiguously concluded the configuration as C14-R for both the isomers 98b and 98c synthesized. The opposite optical activity of the synthesized isomer was compared to the natural product and confirmed the S configuration for the natural product.
2 (Z/E) mixtures of iodo compound 103 to afford a mixture of diastereomers 98b and 98c separable on column purification. The detail spectroscopic analysis unambiguously concluded the configuration as C14-R for both the isomers 98b and 98c synthesized. The opposite optical activity of the synthesized isomer was compared to the natural product and confirmed the S configuration for the natural product.
 | ||
| Scheme 23 Reagents and conditions: (a) ref. 40 quant.; (b) ClCOOCH2CH3, Et3N, THF, −25 °C, 30 min, 71%; (c) TMSCHN2, CH3CN, 0 °C to rt, 6–19 h, 86%; (d) MeOH, C6H5CO2Ag, Et3N, sonication, 1 h, 83%; (e) H2 (balloon), 10% Pd/C, MeOH, rt, 1 h, 78%; (f) Ag2CO3, CH3CN, rt, 36 h, 57%. | ||
Zhai and co-workers undertook an expeditious synthesis of two marine natural products villatamines A 104a and villatamines B 104b (Scheme 24),42 isolated from the extract of the flatworm Prostheceraeus vittatus,43 using proline as a starting material and confirmed the (S) configuration for the naturally occurring isomers. The useful intermediate 105a was successfully prepared from proline according to the reported procedure.44 The compound 105a was reduced using DIBAL and subsequently converted to the terminal alkyne 106 by treating with Bestmann reagent prepared in situ.
 | ||
| Scheme 24 Reagents and conditions: (a) ref. 44; (b) (i) DIBAL-H, toluene, −78 °C; (ii) AcCH2P(O)(OMe)2, TsN3, K2CO3, CH3CN, MeOH, rt; (63% for 2 steps); (c) n-BuLi, THF, −78 °C; ZnBr2, −78 °C; 107, Pd(PPh3)4, THF, rt; (d) 109, Pd(PPh3)4, KOH, H2O, THF, 60 °C, (66% for two steps); (e) (i) p-TSA, MeCN, rt; (ii) CH3CHO, HCl, MeOH, Na(OAc)3BH, rt; (62% for two steps); (f) (i) DIBAL-H, toluene, −78 °C; (ii) CrCl2, CHI3, THF, dioxane, 0 °C–rt; (65% for two steps); (g) 112, Pd(PPh3)4, KOH, H2O, THF, 60 °C, 91%; (h) (i) TSA, MeCN, rt; (ii) CH3CHO, HCl, MeOH, Na(OAc)3BH, rt, (63% for two steps). | ||
The compound 106 on Zn metallation followed by Pd (PPh3)4-catalyzed Negishi coupling with 107 produced bromoenyne 108 which on subsequent Suzuki coupling with 109 afforded 110. Finally the Boc group was deprotected using p-TSA in CH3CN and the free NH was ethylated using reductive amination with CH3CHO in the presence of NaBH(OAc)3 to afford the natural product villatamine A 104a.
For the synthesis of another isomer, the DIBAL reduced product of 105b was subjected to Takai olefination to afford alkenyl iodide 111 which on Suzuki coupling with 112 produced the conjugated alkene compound 113. The synthesis of villatamine B 104b was then completed by deprotection of the Boc group followed by ethylation of the free NH as had been done earlier.
A short synthesis of 2-substituted pyrrolidine alkaloid, (R)-bgugaine 114 was achieved in our laboratory starting from L-proline using the existing (S) chiral centre (Scheme 25).45 The Wittig condensation of 12c46 with in situ prepared phosphorane of 115 gave the olefin 116 which on hydrogenation followed by LAH reduction afforded the natural product (R)-bgugaine 114.
 | ||
| Scheme 25 Reagents and conditions: (a) ref. 46; (b) n-BuLi, THF, 115, 0 °C to rt, 50%; (c) (i) H2–Pd (C), EtOH; (ii) LAH, THF, reflux, 80%. | ||
Clayden and co-workers introduced a short and concise synthesis of (−)-(S,S)-clemastine 117 by using 121 prepared by 1-carbon homologation of Cbz-proline through Arndt–Eistert method (Scheme 26).47 The compound 119 was converted to the requisite chloro compound 121 by LAH reduction of the benzylcarbamate followed by treatment with SOCl2. The labile compound 121 was immediately reacted with 122 to give a mixture of isomers 124, 125 and 126. After several experiments the mixture of 125 and 126 was successfully separated by transforming them to fumerates and by recrystallisation to give enentiomerically pure 117.
Konno and co-workers synthesized trans and cis dendrochrysanine 132,48 Chinese traditional medicines, isolated by Wang and co-workers in 2005 from the stems of Dendrobium chrysanthum.49 The synthetic strategy utilized the homologated Cbz-protected prolinal 127 synthesized from L-proline through conventional synthetic sequences. The aldehyde 127 on Grignard reaction followed by Cbz deprotection and subsequent condensation with trans-cinnamic acid and oxidation of the secondary alcoholic unit using Jones oxidation afforded trans-dendrochrysanine 132a. Similarly treatment of alcohol 129 with cis-cinnamic acid and further conversion gave cis-dendrochrysanine 132b (Scheme 27).
3. Synthesis of pyrrolizidine alkaloids
3.1. Introduction
Pyrrolizidine alkaloids (PAs) bearing an azabicyclic [3,3,0] octane structural motif, are a large family of natural products endowed with vast array of pharmacological and biological properties.50 These alkaloids are generally isolated from flowering and leguminous plants while few have been found in frogs, moths, ants and butterflies.51 The vast range of alkaloids ranging from simple to highly substituted have been found in nature. Manifolds of polyhydroxy PAs are used as potential sugar mimics and have been extensively studied for their potent glycosidase inhibitory activities, making them good candidates as new drugs for the treatment of several diseases like cancer, viral infections and diabetes.52 Proline can contribute to the synthesis of PAs with suitable transformation on the 2nd position and subsequent 5 member cyclisation with amino group.3.2. Unsubstituted pyrrolizidines
The simplest unsubstituted naturally occurring pyrrolizidine alkaloid is pyrrolam, though structurally a pyrolizidinone, included in the class of pyrrolizidine alkaloids. Pyrrolam was isolated in 1990 from Streptomyces olivaceus53 in 4 different structurally related pyrrolizidinone forms, pyarrolam A–D. Of these pyrrolam A attracted considerable attention due to its biological activities and the presence of a double bond responsible for its carcinogenic and mutagenic nature.54 Pyrrolam A, a labile alkaloid, has been synthesized in six different ways starting from proline.The first synthesis of (R)-pyrrolam (A) 133a was achieved by Yuhara and co-workers (Scheme 28) through an intramolecular coupling reaction between a bromoalkyl and ynamide group, assisted by SmI2.55 Initially the (R)-proline was reduced with LAH to prolinol and further converted to 135 either by condensing with phenylpropiolic acid or by converting first to the cyclic carbamate 134 and then reacting with lithiumphenylacetylide. The major precursor bromo compound 136 was prepared by reacting alcohol 135 with NBS and PPh3. The SmI2 intramolecular cyclization was then best achieved using the additive HMPA at 0 °C to afford the exo-cyclic compound 137. The olefinic part of 137 was ozonolysed to afford the diketo compound 138 which was further converted to triflate 139. The triflate 139 was subsequently hydrogenolysed using Bu3SnH to afford pyrrolam (A) 133a.
The synthesis of enantiomerically pure (−)-pyrrolam A 133a was accomplished by Giovenzana et al. in six steps starting from (R)-prolinol with an overall yield of 30% (Scheme 29).56 The Boc protected prolinol 68b was converted to 140 by Mitsunobu dehydrative alkylation protocol. The deprotection of Boc group, triester hydrolysis and cycloamidation were carried out in an one-pot manner without isolating the intermediate to furnish the lactam 142. The installation of the double bond regioselectively by treatment with PhSeCl in the presence of strong base LDA followed by oxidation with H2O2 afforded pyrrolam A 133a.
(+)-Pyrrolam A 133b was successfully synthesized by Arisawa et al. using RCM as a key step (Scheme 30).57 The strategy utilized the Boc-proline converted to alkene 24a by classical synthetic steps. The TFA mediated deprotection of Boc group of 24a and concomitant amidation with methyl acryloyl chloride gave the key intermediate 144. RCM was then successfully performed using Grubbs II catalyst by stirring it for 3 h in benzene at 50 °C affording the pyrrolam A 133b in 30% yield. The low yield was observed due to the instability of the product 133b under the reaction conditions.
Two synthetic routes for (S)-pyrrolam A 133b were developed in our lab by Majik et al. through intramolecular and intermolecular Wittig reaction using L-proline as a chiral source (Scheme 31).58 For the intermolecular route, alkene 146 was prepared by an one-pot oxidation and Wittig reaction of Cbz-prolinol 72 with phosphorane 145. The concomitant deprotection of the Cbz group and reduction of the double bond afforded the cyclized product 147. The required double bond was regioselectively established by treatment of 147 with PhSeCl and H2O2 to render the alkaloid pyrrolam A 133b. The intramolecular version was achieved by converting the prolinol to acetyl bromide protected 148 followed by oxidation with PCC to afford aldehyde 149. The further treatment with PPh3 followed by in situ condensation of phosphorane 150 afforded pyrrolam A 133b.
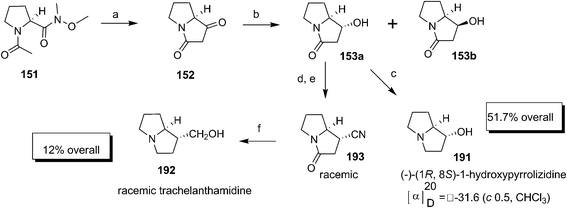
An approach made by Murray and Proctor59 involved cyclization of Weinreb amide 151, prepared from L-proline, to dione 152. The compound 152 on diastereoselective reduction with NaBH4 provided the alcohol 153 as a separable diastereomeric mixture of 153a and 153b (95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5). The compound 153a on mesylation followed by treatment with Et3N installed the double bond regioselectively to afford pyrrolam A 133b (Scheme 32).
5). The compound 153a on mesylation followed by treatment with Et3N installed the double bond regioselectively to afford pyrrolam A 133b (Scheme 32).
 | ||
| Scheme 32 Reagents and conditions: (a) LDA or LHMDS, THF, −78 °C; (b) NaBH4, EtOH, rt, 24 h, (69% for two steps); (c) (i) MsCl, Et3N, DCM, 0 °C–rt, 5 h, 85%; (ii) Et3N, CHCl3, reflux, 5 h, 98%. | ||
The strategy developed by Schobert et al.60 involves the reaction of proline derived benzyl prolinate 154 with the polymer supported cumulated ylide 155 to give the corresponding amide 156. The hydrogenolytic debenzylation of 156 gave the dicarbonyl key intermediate 157 which on subsequent reduction gave alcohol 158. The mesylation of 158 followed by elimination established the double bond to afford pyrrolam 133a (Scheme 33).
3.3. Simple substituted pyrrolizidines
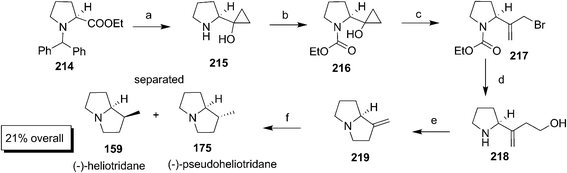
The synthesis of methyl substituted pyrrolizidine alkaloids (−)-heliotridane 159 and (−)-isoretronecanol 160 was successfully accomplished by Knight and Ley using commercially available (S)-N-Boc proline (Scheme 34).61 The Boc-proline was converted to the keto compound 161 through the formation of Weinreb amide followed by Grignard reaction with MeMgI. The keto compound 161 on Wittig reaction with CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) PPh3 afforded the alkenated product 162. The hydroxyl product 163 was prepared by SeO2 oxidation of 162 which on subsequent Boc deprotection of NH and reaction with CH3COCl produced the cyclic compound 164. The key intermediate 166 was synthesized by converting 164 to π-allyltricarbonyliron lactam complex 165 by reacting with diiron nonacarbonyl in benzene under ultrasonication followed by the exhaustive carbonylation under high pressure. The (−)-isoretronecanol 160 was synthesized from 166 by reduction of amide and hydroxylation of alkene using borane. The intermediate 166 was hydrogenated to produce the separable diastereomers 167a and 167b from which 167a upon LAH reduction afforded the natural product (−)-heliotridane 159.
PPh3 afforded the alkenated product 162. The hydroxyl product 163 was prepared by SeO2 oxidation of 162 which on subsequent Boc deprotection of NH and reaction with CH3COCl produced the cyclic compound 164. The key intermediate 166 was synthesized by converting 164 to π-allyltricarbonyliron lactam complex 165 by reacting with diiron nonacarbonyl in benzene under ultrasonication followed by the exhaustive carbonylation under high pressure. The (−)-isoretronecanol 160 was synthesized from 166 by reduction of amide and hydroxylation of alkene using borane. The intermediate 166 was hydrogenated to produce the separable diastereomers 167a and 167b from which 167a upon LAH reduction afforded the natural product (−)-heliotridane 159.
Synthesis of (−)-trachelanthamidine 168 was achieved by Ishibashi et al. via ruthenium catalyzed chlorine atom transfer cyclization using proline as a chiral source (Scheme 35).62 The aldehyde 12c prepared from prolinol was subjected to Wittig olefination to afford alkene 169. The NH group was deprotected and further protected with methyl thio acetyl chloride to give 170. The regioselective chlorination of 170 was accomplished using NCS to provide 171 which on cyclisation performed using RuCl2(PPh3)3 by heating at 140 °C in benzene solution in a sealed tube afforded the bicyclic lactams 172 after removing the minor isomers by column purification. The compound 172 was subjected to nuecleophilic substitution of Cl by CsOCOEt to give 173 which underwent desulfurization on treatment with RANEY® nickel to render 174. The LAH reduction of lactam 174 afforded the natural product (−)-trachelanthamidine 168.
 | ||
| Scheme 35 Reagents and conditions: (a) a–d ref. 63; (e) RuCl2(PPh3)3, 140 °C (sealed tube), 59%; (f) CsOCOEt, DMF, 80 °C, 1 h, 50%; (g) RANEY® nickel, EtOH, reflux, 2.5 h, 86%; (h) LAH, THF, reflux, 5 h, 88%. | ||
Seijas et al. described the synthesis of (−)-pseudoheliotridane 175 and (−)-trachelanthamidine 168, using radical cyclization (Scheme 36).64 The strategy utilized Cbz protected prolinol 72, prepared by reacting Cbz-proline with ClCOOEt with concomitant reduction using NaBH4. The alcohol 72 was oxidised and further converted to alkene 176 using Wittig olefination with PPh3![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2. The Cbz group was hydrogenolysed and further protected with Cl3CCOCl to afford 177. The earlier Cbz protection was necessary since Cl3CCOCl group was labile under NaBH4 conditions. The radical cyclization of chorocompound 177 took place by refluxing with CuCN in CH3CN in a sealed tube. The reaction was highly diastereoselective affording only 178 due to steric hindrance of the pyrrolidine nucleus. The trichloro compound 178 was further converted to monochloro compound 179 under catalytic hydrogenation condition. The nucleophilic substitution of Cl of 179 by I furnished 180 which could conveniently be transformed to the aforementioned natural products.
CH2. The Cbz group was hydrogenolysed and further protected with Cl3CCOCl to afford 177. The earlier Cbz protection was necessary since Cl3CCOCl group was labile under NaBH4 conditions. The radical cyclization of chorocompound 177 took place by refluxing with CuCN in CH3CN in a sealed tube. The reaction was highly diastereoselective affording only 178 due to steric hindrance of the pyrrolidine nucleus. The trichloro compound 178 was further converted to monochloro compound 179 under catalytic hydrogenation condition. The nucleophilic substitution of Cl of 179 by I furnished 180 which could conveniently be transformed to the aforementioned natural products.
 | ||
Scheme 36 Reagents and conditions: (a) (i) Et3N, ClCOOEt; (ii) NaBH4 (78% for two steps); (b) (i) Swern, 98%; (ii) PPh3![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2, 51%; (c) (i) HBr, AcOH; (ii) Cl3CCOCl, DMAP (82% for two steps); (d) CuCl, CH3CN, 150 °C, 93%; (e) H2, Pd/C, 96%; (f) NaI, 81%; (g) (i) H2, Pd/C, Et3N, 86%; (ii) LAH, THF, reflux (ref. 65) 68%; (h) (i) AgOAc; (ii) LAH (ref. 65) (77% for two steps). CH2, 51%; (c) (i) HBr, AcOH; (ii) Cl3CCOCl, DMAP (82% for two steps); (d) CuCl, CH3CN, 150 °C, 93%; (e) H2, Pd/C, 96%; (f) NaI, 81%; (g) (i) H2, Pd/C, Et3N, 86%; (ii) LAH, THF, reflux (ref. 65) 68%; (h) (i) AgOAc; (ii) LAH (ref. 65) (77% for two steps). | ||
Taddei and co-workers disclosed the synthesis of (−)-heliotridane 159, (−)-pseudoheliotridane 175, (−)-isoretronecanol 160 and (−)-trachelanthamidine 168 through diastereoselective Michael addition of alkyl cuprate to γ-aminocunjugated alkene (Scheme 37).66 The synthetic strategy utilized the conversion of (S)-Boc-proline to aldehyde 12b through the formation of Wienreb amide followed by LAH reduction. The olefinic compound 181 prepared by Wittig olefination was subjected to Michael addition with methyl cuprate and vinyl cuprate to afford the diastereomeric mixture 182 and 183 respectively. The mixture 182 was subjected to cyclization to give lactams 167a and 167b which were separable by flash chromatography. The synthesis of (−)-heliotridane 159 and (−)-pseudoheliotridane 175 was then furnished by LAH reduction of the lactams 167a and 167b respectively. Similarly the vinylated compound 183 was transformed to diastereomeric mixture 184 which was further converted to cyclic esters 185 and 186. The compounds 185 and 186 were further reduced to the natural products (−)-isoretronecanol 160 and (−)-trachelanthamidine 168 respectively, using LAH.
 | ||
Scheme 37 Reagents and conditions: (a) TEA, pivaloyl chloride, Me(NH)(OMe), 89%; (b) LAH, 0 °C, 96%; (c) PPh3![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2COOMe, THF, rt; (d) R2CuLi, TMSCl, −30 °C; (e) HCl, AcOH; (f) pyridine, DMAP, reflux; (g) flash chromatography; (h) LAH, reflux; (i) ref. 67. CH2COOMe, THF, rt; (d) R2CuLi, TMSCl, −30 °C; (e) HCl, AcOH; (f) pyridine, DMAP, reflux; (g) flash chromatography; (h) LAH, reflux; (i) ref. 67. | ||
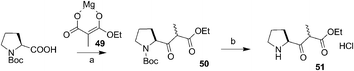
Hassner et al. achieved the synthesis (−)-supinidine 187 by applying intramolecular oxime-olefin cycloaddition (Scheme 38).68 The unstable vinyl compound 188 prepared from proline was converted to oxime 189 which on heating at 180 °C afforded the cyclic product 190 along with some by-products. The compound 190 on reductive cleavage with LAH followed by diazotisation afforded the natural product (−)-supinidine 187.
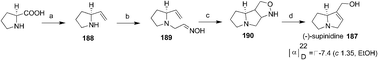 | ||
| Scheme 38 Reagents and conditions: (a) and (b) ref. 69; (c) 180 °C, 15 h, 56%; (d) (i) LAH, 87%; (ii) 2N HCl, NaNO2, 0 °C, 53%. | ||
Murray and Proctor continued their earlier developed strategy, N-acyl anion cyclisation for the synthesis of some of the naturally occurring pyrrolizidines like (−)-(1R,8S)-1-hydroxypyrrolizidine 191 and (±)-trachelanthamidine 192 (Scheme 39).70 The successful N-acyl anion cyclisation was ventured with optimal use of either LDA or LHMDS at −78 °C on N-methoxy-N-methyl amide 151 prepared from L-proline. Thus the Wienreb amide 151 cyclised to afford the diketo compound 152 with a very slight racemisation. The highly selective diastereofacial reduction with NaBH4 afforded the mixture of diastereomers 153a and 153b. The synthesis of (−)-(1R,8S)-1-hydroxypyrrolizidine 191 was achieved by direct LAH reduction of 153a. The major isomer 153a was then mesylated and further treated with NaCN to afford cyano compound 193, but surprisingly with a complete loss of enantiomeric purity. The compound (±)-trachelanthamidine 192 was then prepared on methanolysis of 193 followed by LAH reduction.
With continuing interest in radical cyclization and its applications to pyrrolizidines, Ishibashi et al. recently reported the synthesis of (−)-trachelanthamidine 168 through their well developed single electron transfer strategy (Scheme 40).71 The requisite alkene 195 was prepared by Julia olefination of Boc-prolinal 12b with α-benzyloxy sulfone 194 which on deprotection of the Boc group afforded 196. The compound 196 on trichloroacetylation gave 197 which was subjected to cyclisation by refluxing with 1,4-dimethylpiperazine. The surprising failure of the method to give the product 198 turned the attention to prepare 200 through the formation of aldehyde 199. The compound 200 underwent expected cyclisation affording the product 201 which on dechlorination gave the product 202. The targeted compound (−)-trachelanthamidine 168 was achieved by direct LAH reduction of 202.
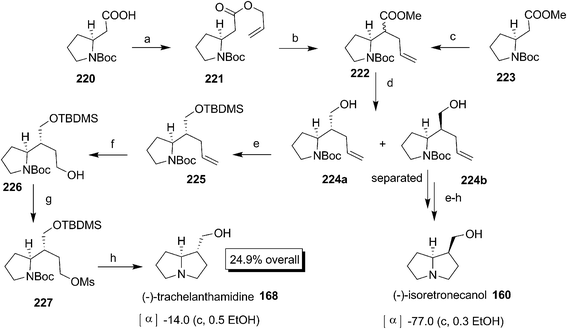
Reddy et al. succeeded in the formal synthesis of (−)-isoretronecanol 160 and (−)-trachelanthamidine 168 starting from proline using ring closing metathesis (Scheme 41).72 The alkene 24a was prepared according to the well developed procedure from ethyl ester of Boc-proline 23 which on dihydroxylation followed by the protection of terminal –OH afforded 203. The compound 203 was oxidised to ketone 204 and subjected to Wittig olefination to give 205. The deprotection of the Boc group followed by reaction with acryloyl chloride afforded the ready intermediate 206 for RCM. The RCM of 206 using Grubbs II catalyst gave compound 207 which on hydrogenation followed by benzoylation afforded a separable mixture of 208a and 208b. The deprotection of benzoyl group of 208a and 208b gave 209a and 209b respectively which constituted the formal synthesis73 of (−)-isoretronecanol 160 and (−)-trachelanthamidine 168.
Craig and co workers successfully synthesized (−)-trachelanthamidine 168 using Pd catalysed intramolecular cyclisation (Scheme 42).74 The methyl ester of Boc proline 67 was converted to allyl alcohol 210 by DIBAL reduction with subsequent Wittig reaction. After several experiments the ester 211 was subjected to Pd catalysed cyclisation successfully delivering the products 212 with 212b as the major isomer after purification. The olefin 212b was transformed to 213 by reductive ozonolysis which on subsequent detosylation uneventfully produced the lactam 209b whose relative configurations were assigned by X-ray crystallography. The LAH reduction of 209b gave the natural product 168.
Kulinkovich and Lysenko accomplished the synthesis of (−)-heliotridane 159 and (−)-pseudoheliotridane 175 using cyclopropanation of the ester group using titanium mediated Grignard reaction (Scheme 43).75 The synthesis commenced with the cyclopropanation of proline ester 214 with Ti(OPr)4 in the presence of 3.0 equiv. of Grignard reagent, followed by hydrogenolysis to afford 215. After protecting the free NH with chloroformate, the compound 216 was subjected to mesylation in the presence of MgBr2 to give 217. The compound 217 on Reformatsky reaction with formaldehyde produced 218 which underwent cyclisation to afford 219 under Mitsunobu condition. The hydrogenation of 219 in the presence of NiCl2–NaBH4 gave a mixture of 159 and 175 (11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) separable by column purification.
1) separable by column purification.
Knight and co-workers synthesized (−)-trachelanthamidine 168 and (−)-isoretronecanol 160 (Scheme 44).76 Claisen rearrangement of ester 221 prepared from Boc-homoproline 220 gave and inseparable diastereomeric mixture of 222. The ester 222 was also directly obtained from from Boc-homoproline methyl ester 223. The DIBAL reduction of 222 gave a separable mixture of 224. The less polar erythro isomer 224a was successfully transformed to 168 through classical synthetic sequences. In a similar way the more polar threo 224b was converted to 160.
3.4. Hydroxylated pyrrolizidines
Shanyoor and Mulzer revealed a synthesis of (−)-petasinecine 228 through Ireland–Claisen type rearrangement (Scheme 45).77 Initially Boc-proline methyl ester 67 was converted to allylic alcohol 210 by following a literature report.78 The allyl ester 229 prepared by treatment of 210 with benzoxyacetoyl chloride was subjected to Claisen rearrangement using TMSCl and LiHMDS at −110 °C to afford the compound 231 as the only diastereomer through the intermediacy of 230. The reductive ozonolysis of 231 with subsequent borane reduction followed by hydrogenolysis furnished the natural alkaloid 228.The naturally occurring alkaloid 1-hydroxyprrolizidine 233 was synthesized by Guerreiro et al. using diastereofacial hydrogenation of carbonyls using chiral ligands (Scheme 46).79 The compounds 234a and 234b were synthesized from L-proline and R-proline respectively using the literature methods.80 The reduction of the carbonyl with chiral ligands complexed with Ru(II) under hydrogenation displayed the concept of matched and mismatched pairs. The selectivity was determined by two factors, the chirality of the proline moiety and the chirality of the ligand complexed with ruthenium. Thus the ideal case for matched pair was when (S)-234a gave diastereoselectively 235a with (R)-BINAPRu(II) and (R)-MeO-BIPHEPRu(II) while (R)-234b gave diastereoselectively 235c with (S)-BINAPRu(II). The mismatching was observed for the opposite stereoisomers. The optically pure 235c was then subjected to Boc deprotection and subsequent intramolecular cyclization leading to the synthesis of optically pure 1-hydroxypyrrolizidine 233.
Synthesis of the deoxy congener 238b of the pyrrolizidine alkaloid hyacinthacine 238a was achieved by Izquierdo et al. via indium mediated diastereoselective addition of allyl indium bromide to Cbz-prolinal 12a (Scheme 47).81 The compound 236 obtained was subjected to epoxidation using MCPBA to afford 237 whose structure was elucidated by different spectroscopic techniques. Further, catalytic hydrogenolysis of Cbz gave the cyclised product 238b which was isolated by acetylating with acetic anhydride as 238c for characterisation purposes.
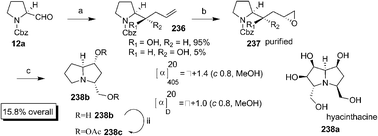 | ||
Scheme 47 Reagents and conditions: (a) CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCH2InBr, THF, −78 °C, 78%; (b) MCPBA, DCM, rt, 75%; (c) (i) 10% Pd/C, H2; (ii) Ac2O, pyridine, rt (27% two steps). CHCH2InBr, THF, −78 °C, 78%; (b) MCPBA, DCM, rt, 75%; (c) (i) 10% Pd/C, H2; (ii) Ac2O, pyridine, rt (27% two steps). | ||
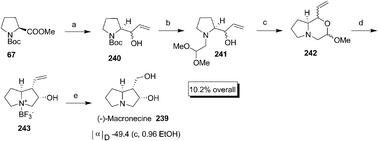
Ito et al. synthesized (−)-macronecine 239 during the synthetic use of zirconium mediated diastereoselective ring contraction of vinyl morpholine derivatives prepared from amino acids.82 The proline based morpholine derivative 242 prepared from Boc-proline was reacted with “Cp2Zr” in the presence of BF3·OEt2 to afford pyrrolizidine–BF3 complex 243 as a single diastereomer which on subsequent reductive ozonolysis and neutralisation gave 239 (Scheme 48).
3.5. Miscellaneous examples
Duarte et al. utilized L-proline as a starting material for the solid phase synthesis of 248 and claimed it as a core unit for hyacinthacine 238a (Scheme 49).83 The Boc-proline was coupled with Merrifield resin 244 to afford 245. The amino salt 246 obtained by the deprotection of Boc was subjected to Michael reaction with ethyl propiolate to give 247. The core unit 248 was then prepared by successful Baylis–Hillman reaction of 247 using DBU under microwave irradiation in 37% yield. | ||
| Scheme 49 Reagents and conditions: (a) K2CO3, KI, reflux, 24 h; (b) DCM, Et2O, 3 M HCl; (c) DIPEA, DMF, 4 days, rt; (d) DBU, MW, 10 min. | ||
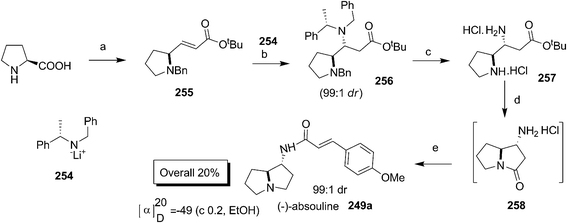
Synthesis of 1-aminopyyrolizidine alkaloid (−)-absouline 249a was accomplished by Scheerer and co-workers using conjugate addition of amines to the unsaturated ester derived from proline (Scheme 50).84 The authors carried out several studies to improve the heterocunjugate addition of amine to 250a and 250b by varying the solvents, reaction conditions and bases in producing an inseparable mixture of 251a and 251b which as such was subjected to cyclization to afford a separable mixture of lactams 252a and 252b. The absolute configuration was eventually established by X-ray analysis of salt of 252. The synthesis of the alkaloid 249a was then achieved by reduction of 252 to amine 253 followed by DCC coupling with 4-methoxycinnamic acid.
In continuation of highly diastereoselective conjugate addition studies of lithium–amide based chiral auxiliaries for the synthesis of natural products,85 Davies et al. have recently reported the synthesis of (−)-(1R,7a,S)-absouline 249a from L-proline (Scheme 51).86 The conjugate addition of lithium (S)-N-benzyl-N-(α-methylbenzyl) amide 254 to 255 took place diastereomerically to give 256. The hydrogenation of 256 followed by treatment under strong acidic conditions gave the cyclised amide 258 from 257. The DIBAL reduction of 258 followed by subsequent condensation of the free amine with trans-4-methoxycinnamic acid afforded the natural product (−)-absouline 249a.
Christine et al. achieved the synthesis of laburnamine and absouline along with their epimeric congeners.87 The ester 259 prepared from proline was transformed to cyclic amines 262a and 262b in racemic forms which on condensation with the corresponding acid chlorides afforded the natural products absouline 249b, c and labunamine 263a, b in both the diastereomeric forms (Scheme 52).
 | ||
| Scheme 52 Reagents and conditions: (a) (i) NaOEt, xylene; (ii) H+, reflux; (b) NH2OH, HCl; (c) Na–NH3 or H2–PtO2. | ||
4. Synthesis of indolizidine alkaloids
4.1. Introduction
Indolizidine alkaloids are comprised of a [4.3.0] azabicyclic nonane core, present in the numerous bioactive natural and unnatural scaffolds.88 They are mainly isolated from skin secretions of amphibians.89 This has attracted interest from synthetic chemists due to their potent biological and medicinal applications. Coniceine is the simplest indolizidine with unsubstituted 5-member and six member rings fused to each other. The indolizidine moieties even with alkyl substitution at various positions exhibit unique characteristics especially in blocking neuromuscular transmission.90 The polyhydroxy substituted indolizidines like swainsonine, castanospermine alkaloids have attracted special interest for their anti HIV and anticancer properties and are also known for being the best mimics of sugars to act as potential glycosidase inhibitors.91 The formulation of indolizidine alkaloids can be achieved either by starting with a six member heterocycle and then annulating a five member on to it or vice versa. The proline being a 5 member heterocycle can efficiently be used for the construction of indolizidines by appropriate manipulation of the side chain and wrapping it to form a six member ring around it.4.2. Unsubstituted indolizidines (coniceine)
Sibi and Christensen formulated the synthesis of (−)-δ-coniceine 264 from Boc-proline (Scheme 53).92 The Boc-prolinal 12b was prepared using conventional steps which on Wittig reaction with phosphorane of 265 afforded the olefin 266. The hydrogenation of 266 followed by mesylation of hydroxyl group gave 267. The compound 267 on Boc deprotection with subsequent neutralization furnished δ coniceine 264.Nakagawa's group synthesized (−)-coniceine 264 using RCM as a key step (Scheme 54).57 The method is similar to the one described earlier (Scheme 30) for pyrrolam. The compound 268 was subjected to RCM using Grubbs II catalyst to afford 269 which on hydrogenation furnished 270. The LAH reduction of 270 gave (−)-coniceine 264.
Chang and co-workers encompassed an efficient formal synthesis of (−)-coniceine 264 (Scheme 55)93 using RCM strategy, similar to Nakagawa’s approach. The only difference lies in nature of the side chain and the group attached to N atom. The HCl salt of methyl proline ester was converted to iodo compound 271 from alcohol 51a with conventional synthetic sequences. The treatment of the compound 271 with vinylmagnesium bromide in the presence of CuI gave 272 which on deprotection of the –Boc afforded amine 273. The key intermediate for RCM 274 was prepared by incorporating an acryloyl double bond over free –NH of 273. The RCM on 274 with Grubbs II catalyst furnished 269 which on double bond reduction gave the lactam 270, completing the formal synthesis of (−)-coniceine 264.94
Recently Pinho and Burtoloso approached formal syntheses of (−)-coniceine 264 by employing an unusual Wolf rearrangement (Scheme 56).95 The synthesis commenced with Horner–Wittig condensation of the phosphonate 275 with Cbz-prolinal 12a to furnish 276. The problems encountered under various conditions tried for Wolf rearrangement of 276 were circumvented by employing a photochemical condition which without any epimerisation at the chiral centre afforded 277 in 97% yield. The compound 277 underwent cyclization to azabicyclic lactam 270 by hydrogenolysis of Cbz group and concomitant reduction of the double bond to complete the formal synthesis94 of (−)-coniceine 264.
 | ||
| Scheme 56 Reagents and conditions: (a) NaH, THF, −78 °C, 70%; (b) MeOH, hν, 25 °C, 4 h, 97%; (c) Pd/C, MeOH, Et3N, 48 h, 25 °C, 92%. | ||
4.3. Simple substituted indolizidines
Lhommet and co-workers explored the synthesis of three different substituted indolizidine alkaloids namely, (−)-195B 278, (−)-239AB 279, (−)-223AB 280, using proline as an original chiral source by synthesizing a versatile common intermediate 285 (Scheme 57).96 The pivotal steps involve the diastereoselective metal mediated coupling at the C-5 of the pyrrolidine and the reductive amination of the imine formed in situ. Initially the Cbz-proline ester was methoxylated at C-5 using anodic oxidation, a method developed by T. Shono,97 to afford 281. The compound 281 on BF3 mediated coupling with pent-4-enyl copper succeeded with high diastereoselectivity to afford 282 (trans![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cis/96
cis/96![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) which was subjected to chemoselective reduction to alcohol 283 to separate as a single isomer. The compound 283 was tosylated to 284 and further, the homologation was achieved by the nucleophilic displacement of OTs by reacting with excess of n-Pr2CuLi to give the key building block 285.
4) which was subjected to chemoselective reduction to alcohol 283 to separate as a single isomer. The compound 283 was tosylated to 284 and further, the homologation was achieved by the nucleophilic displacement of OTs by reacting with excess of n-Pr2CuLi to give the key building block 285.
The synthesis of aforementioned indolizidines was achieved by a systematic transformation of the olefinic part of 285.
The synthesis of 278 was furnished by Wacker oxidation of 285 followed by hydrogenolysys of Cbz group.
For the synthesis of 280 the olefinic part of 285 was epoxidised to 287 and further treated with excess Grignard reagent EtMgBr to give a diastereomeric mixture 288 which on direct oxidation gave the compound 289. The hydrogenolysis of 289 as earlier afforded the compound 280 along with its separable epimer.
For the synthesis of 279 the epoxide 287 was treated with excess of vinylmagnesium bromide to afford a diastereomeric mixture 290. The mixture 290 was as such oxidised followed by the hydroboration oxidation of the terminal double bond and protection as benzoyl group gave 291. The compound 291 on hydrogenolysis and subsequent benzoyl deprotection of the resultant 292 provided the indolizdine 279.
Gang and co-workers synthesized indolizidines (−)-209D 293 and 209B 294 (Scheme 58).98 The nucleophilic addition of ethyl propiolate anion to carbonyl of 12a afforded a mixture of diastereomers 295 which on hydroxyl protection with TBSCl afforded 296. The subsequent hydrogenation of 296 over Pd/C in MeOH contributed the deprotection of carbamate, reduction of triple bond and the cyclisation to lactam to take place in one pot affording a mixture of 297a and 297b in a ratio of 1.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, separated by column chromatography. The compound 297a (297b) was then treated with C6H13MgBr followed by iminium ion reduction afforded single isomer 298a (298b), the stereochemical control was attributed to the less hindered α-H atom of pyrrolidine ring which favoured the formation of β-isomer. The TBS group of 298a (298b) was deprotected in acid condition to afford 299a (299b). The successful synthesis of 293 was reached by converting the hydroxyl group of 299a (299b) to thiocarbamate ester 300a (300b) and then by deoxygenation with Bu3SnH under Barton–McCombie deoxygenation conditions.
1, separated by column chromatography. The compound 297a (297b) was then treated with C6H13MgBr followed by iminium ion reduction afforded single isomer 298a (298b), the stereochemical control was attributed to the less hindered α-H atom of pyrrolidine ring which favoured the formation of β-isomer. The TBS group of 298a (298b) was deprotected in acid condition to afford 299a (299b). The successful synthesis of 293 was reached by converting the hydroxyl group of 299a (299b) to thiocarbamate ester 300a (300b) and then by deoxygenation with Bu3SnH under Barton–McCombie deoxygenation conditions.
For the synthesis of indolizidine 209B 294, the keto compound 301 was subjected to nucleophilic addition of lithiopropiolate-ion to afford a mixture of 302a and 302b (2.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) separable by column chromatography. The mixture 302 was subjected to hydrogenation furnishing 303a and 303b which was as such dehydrated to give the olefin 304. The hydrogenation of 304 under high pressure afforded an inseparable mixture of 305a and 305b which upon addition of C5H11MgBr followed by iminium ion reduction gave the pure indolizidine 294 after column purification.
1) separable by column chromatography. The mixture 302 was subjected to hydrogenation furnishing 303a and 303b which was as such dehydrated to give the olefin 304. The hydrogenation of 304 under high pressure afforded an inseparable mixture of 305a and 305b which upon addition of C5H11MgBr followed by iminium ion reduction gave the pure indolizidine 294 after column purification.
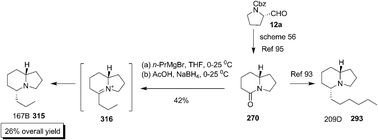
Back and Nakajima developed a method to construct (−)-indolizidine 167B 315 and (−)-indolizidine 209D 293 through conjugate addition of γ-chloroamines 306 to acetylenic sulfones 307 and 308 respectively.99 The deprotonation of chloro compounds 309 (310) using LDA gave cyclised product 311 (312). The reduction of the double bond of 311 (312) using NaCNBH4 and subsequent desulfonation produced required products with a tiny amount of 313 (314). Thus the crude mixture was subjected hydrogenation over Pd/C to afford (−)-indolizidine 167B 315 (209D 293) (Scheme 59).
Pinho and Burtoloso also approached the total synthesis of (−)-indolizidine 167B 315 and formal syntheses of (−)-indolizidine 209D 293 by employing an unusual Wolf rearrangement as described earlier under Scheme 56.95 The synthesis of bicyclic lactam 270 constituted the formal synthesis of (−)-indolizidine 209D 293. The synthesis of 315 was achieved by diastereoselective addition of n-PrMgBr to 270 followed by iminium ion reduction (Scheme 60).
Stereoselective synthesis of (−)-indolizidine 209D 293 was furnished by Ponpandian and Muthusubramanian using sequential deprotection-cyclisation protocol (Scheme 61).100 After overcoming the several consequences of epimerisation and inconvenient routes, the authors emerged with an appropriate sequence to bring about the deprotection and cyclisation of 320 efficiently. The compound 319 was prepared by hydrogenation of 318 which in turn was accessed from Boc-prolinal 12b using Wittig reaction with the phosphorane of the corresponding salt 317. The β-ketoester 320 was prepared by condensing CDI with acid 319 followed by treatment with ethyl potassium malonate in the presence of anhy. MgCl2. The BF3·OEt2 mediated deprotection of the Boc group of 320 with subsequent cyclisation by treatment with NaHCO3 gave the trans olefin 321. The hydrogenation of 321 afforded the pure isomer 322. The LAH reduction of 322 followed by tosylation and subsequent CuI mediated coupling with n-BuLi furnished indolizidine alkaloid 209D 293.
4.4. Hydroxyindolizidines
St-Denis and Chan accomplished the synthesis of all four diastereomers of 1-deoxycastenospermine 330 through diastereoselective addition of anion of allyl phenyl sulphide and Sharpless dihydroxylation (Scheme 62).101 The synthetic strategy utilized 12a obtained from L-proline. The titanium mediated addition of anion of allyl phenyl sulphide 323 to 12a occurred diastereoselectively affording only two isomers 324a and 324b out of four possible isomers. The isomers were separated by column purification and the synthesis was furthered with the major isomer 324a. The oxidation of thio group of 324a followed by allylic rearrangement using P(OMe)3 afforded the allyl alcohol 325 which on treatment with NaOH furnished the cyclic carbamate 326. The compound 326 was chlorinated to give 327 prior to dihydroxylation affording the diastereomers 328a and 328b (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), separated by column chromatography to further the synthesis with the major isomer 328a. The protection of the diol 328a as acetonide group followed by opening up of the carbamate using NaOH afforded the cyclised product 329a. The stereochemistry of the compound was established at this stage through various spectroscopic techniques to confirm the structure of 329a. In a similar way all the isomers 329b, c, d were synthesized by utilizing the other isomers formed during the synthetic sequence. The synthesis of all four diastereomers of 1-deoxycastenospermine 330 (a–d) was then smoothly achieved by the deprotection of the acetonide group of 329 (a–d) using TFA.
1), separated by column chromatography to further the synthesis with the major isomer 328a. The protection of the diol 328a as acetonide group followed by opening up of the carbamate using NaOH afforded the cyclised product 329a. The stereochemistry of the compound was established at this stage through various spectroscopic techniques to confirm the structure of 329a. In a similar way all the isomers 329b, c, d were synthesized by utilizing the other isomers formed during the synthetic sequence. The synthesis of all four diastereomers of 1-deoxycastenospermine 330 (a–d) was then smoothly achieved by the deprotection of the acetonide group of 329 (a–d) using TFA.
Zhang et al. achieved the synthesis of two isomers of 1-deoxy-8a-epi-castanospermine 331a and 331b by diastereoselective addition of ethyl lithiopropiolate and Sharpless dihydroxylation as key steps (Scheme 63).102 The diastereoselective addition of ethyl lithiopropiolate to carbonyl derived from Boc-prolinal 12b in the presence of HMPA afforded the two separable diastereomers 332a and 332b (2.6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). The secondary hydroxyl group of 332a was then protected using TBSCl to afford 333. The selective triple bond reduction to double bond was achieved using Lindlar's catalyst to give olefin 334 which on Boc deprotection using TFA followed by treatment with Et3N furnished the cyclised product 335. The compound 335 was subjected to Sharpless dihydroxylation to give diol 336 which on subsequent reduction with borane gave 337. The deprotection of TBS group of 337 using TBAF produced the natural product 331a whose structural elucidation was done using different spectroscopic techniques. Similar synthetic steps were repeated for the synthesis of the other isomer 331b from 332b.
1). The secondary hydroxyl group of 332a was then protected using TBSCl to afford 333. The selective triple bond reduction to double bond was achieved using Lindlar's catalyst to give olefin 334 which on Boc deprotection using TFA followed by treatment with Et3N furnished the cyclised product 335. The compound 335 was subjected to Sharpless dihydroxylation to give diol 336 which on subsequent reduction with borane gave 337. The deprotection of TBS group of 337 using TBAF produced the natural product 331a whose structural elucidation was done using different spectroscopic techniques. Similar synthetic steps were repeated for the synthesis of the other isomer 331b from 332b.
Koskinen and Kallatsa formulated the synthesis of 1-deoxy-8,8a-di-epi-castanospermine 330a using proline as an efficient starting material (Scheme 64).103 The phosphonate 338 was prepared from Boc-proline ester according to the procedure reported by Heathcock and von Geldern.104 The Horner–Wadsworth–Emmons olefination was then achieved on 338 to afford 339 using mild base K2CO3. The stereoselective reduction of carbonyl of 339 rendered the separable mixture of 340a and 340b. The dihydroxyalation of isomer 340a gave a mixture of 341a and 341b, separated by column chromatography. The major compound 341b was furthered by acetylating the free hydroxyl groups to give 342 and subsequently hydrogenated to give 343. The terminal free OH group of 343 was mesylated to give 344. The compound 344 on Boc deprotection using TFA underwent cyclisation to give 345 which on subsequent treatment with NaOH furnished 330a.
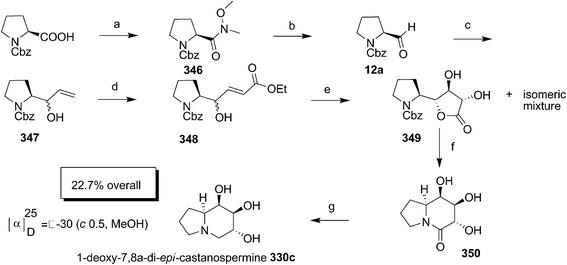
Bhat and co-workers made an entry into the synthesis of castenospermine alkaloid by synthesizing 1-deoxy-7,8a-di-epi-castanospermine 330c through RCM and Upjohn dihydroxylation (Scheme 65).105 The Cbz-prolinal 12a was prepared by condensing the Cbz-proline with methoxy methyl amine chloride followed by LAH reduction of the Weinreb amide 346. The Grignard addition of vinylmagnesium bromide on 12a produced an inseparable mixture of diastereomers 347 which as such subjected to cross olefin metathesis with methyl acrylate in the presence of 2nd generation Grubbs II catalyst to afford 348. The dihydroxylation of 348 gave a mixture of isomers which on purification by column chromatography afforded the major pure isomer 349, the structure of which was confirmed by single X-ray analysis. The diol 349 without prior protection of –OH, hydrogenated to give amide 350 which on subsequent reduction using borane produced the targeted compound 330c.
Bernardim et al. synthesized several castanospermine analogues by synthesizing a robust intermediate 277 (Scheme 66)106 by well known efficient Wolf rearrangement of 276 prepared from phosphonate 275 and Cbz-prolinal 12a under photocatalytic condition. The dihydroxylation of 277 afforded 351 which on subsequent hydrogenation gave 352. The synthesis of 1,6-dideoxy-castenospermine 354 was completed by direct reduction of the lactam 353 using BH3·Me2S. Similarly, the compound 277 on epoxidation followed by treatment with DBU produced a diastereomeric mixture of 356a and 356b (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). The mixture of alkene 356 when dihydroxylated gave a mixture of 357 and 349 (4
1). The mixture of alkene 356 when dihydroxylated gave a mixture of 357 and 349 (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) with facial selectivity. The hydrogenation of 357 provided 358 after purification using column chromatography. The reduction of the lactam 358 afforded castanospermine analogue 1-deoxy-8,8a-di-epi-castenospermine 330a. Interestingly the synthesis of 359a and 359b by hydrogenation of 356a and 356b constituted the formal syntheses of pumiliotoxin 251D 360 (ref. 107) and of octahydroindolizidin-8-ols 361a and 361b.108
1) with facial selectivity. The hydrogenation of 357 provided 358 after purification using column chromatography. The reduction of the lactam 358 afforded castanospermine analogue 1-deoxy-8,8a-di-epi-castenospermine 330a. Interestingly the synthesis of 359a and 359b by hydrogenation of 356a and 356b constituted the formal syntheses of pumiliotoxin 251D 360 (ref. 107) and of octahydroindolizidin-8-ols 361a and 361b.108
Suh and co-workers efficiently applied their ACR-induced stereoselective ring-expansions of lactams for the synthesis of 1-deoxy-6,8a-di-epi-castenospermine 362 and 1-deoxy-6-epi-castenospermine 363 (Scheme 67).109 The 1-carbon homologated Boc prolinal was converted diastereoselectively to 364a and 364b by differential selection of base DBU and NaH respectively. The selective deprotection of 364a using TMSOTf and 2,6-lutidine followed by coupling with protected glycolic acid resulted 365a. The compound 365a on ACR execution afforded the 7 member lactam 366 which on treatment with oxone resulted in the formation of 367 via trans annulations and concomitant TBS deprotection. The formation of 366 was elegantly explained by the authors by invoking different transition states. The triol 367 was acetylated and reduced with LAH to afford 362. In a similar way the cis isomer 364b was transformed to 363 with ACR induced technique.
Cossy and co-workers have accomplished two formal synthesis of (−)-swainsonine 372 by enantioselective ring expansion of prolinol derivatives.110 The ring expansion traverses through the formation of aziridinium ion (Scheme 68) which was first proposed by Fuson and Zirkle in 1948 (ref. 111) and successfully utilised by O'Brien's group.112 The commercially available proline was converted to trityl ester 373 which on LAH reduction followed by Swern oxidation afforded the aldehyde 374. The diastereoselective addition of vinylmagnesium chloride to the aldehyde 374 afforded the hydroxyl derivative 375 diastereoselectively (98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2). In order to obviate the further synthetic consequences, the trityl group was converted to benzoyl to give the compound 376. The compound 376 on treatment with acryloyl chloride produced the compound 377 which on RCM using Grubbs II catalyst afforded the product 378. The syn dihydroxylation of the olefin 378 followed by diol protection provided the compound 379 which on LAH reduction gave 380. When the attempted ring expansion of this 380 was unsuccessful, the compound 381 was prepared by protecting primary –OH with acetyl group and the secondary –OH of 381 was transformed to –Cl under microwave condition to afford 382. The treatment of 382 with AgOAc effected the ring expansion smoothly to give 384 through the formation of aziridinium ion 383. The further conventional synthetic steps performed over 384 gave 385 to complete the formal synthesis of (−)-swainsonine 372 (Scheme 69).113
2). In order to obviate the further synthetic consequences, the trityl group was converted to benzoyl to give the compound 376. The compound 376 on treatment with acryloyl chloride produced the compound 377 which on RCM using Grubbs II catalyst afforded the product 378. The syn dihydroxylation of the olefin 378 followed by diol protection provided the compound 379 which on LAH reduction gave 380. When the attempted ring expansion of this 380 was unsuccessful, the compound 381 was prepared by protecting primary –OH with acetyl group and the secondary –OH of 381 was transformed to –Cl under microwave condition to afford 382. The treatment of 382 with AgOAc effected the ring expansion smoothly to give 384 through the formation of aziridinium ion 383. The further conventional synthetic steps performed over 384 gave 385 to complete the formal synthesis of (−)-swainsonine 372 (Scheme 69).113
The second strategy utilized the hydroxyl allyl intermediate 375 which was converted to N-allylic compound 386, suitable for ring expansion. The ring expansion of 386 was performed in the presence of (CF3COO)2O followed by treatment with NaOH to afford 387 whose OH was further protected with TBDMS to give 388. The RCM on HCl salt of 388 using Grubbs I catalyst gave the compound 389 which completed the formal synthesis of (−)-swainsonine 372 (Scheme 70).114
4.5. Pumiliotoxins
Pumiliotoxins are bicyclic indolizidine alkaloids, isolated from the amphibians Dendrobates pumilio in South America, in 1963.115 They are best known for their cardiotonic activities and myotonic activities.116 Pumiliotoxins 390 and their hydroxyl congener allopumiliotoxins 391 differ by their structure in terms of hydroxyl groups.The designated pumiliotoxins are characterised in the general structural form 390. There have been almost 40 members of this family isolated and found to show potent pharmaceutical behaviours. The (Z)-alkylidene side chain has attracted major attention in contemplating the design of the total synthesis for pumiliotoxins since the incorporation of a stereo controlled exocyclic double bond has been a formidable challenge117 for synthetic chemists. The basic indolizidine core with a 5 member nitrogen stereo centre can be best realized using proline as a starting material. Over the last few years many chiral pool syntheses of these complex molecules have been performed and most of them involved proline as a major synthetic precursor for the construction of the chiral pyrrolidine motif.
Overmann and co-workers pioneered118 the synthesis of heterocycles and pumiliotoxins by using their well developed iodide promoted iminium ion-alkyne cyclization strategy (Scheme 71). The epoxide 392 envisioned to be the viable precursor,119 was synthesized from proline according to the literature reports.120 The diastereoselective opening up of the epoxide 392 by using 2.0 equiv. of alkynylalane 393 afforded 395 exclusively. The Cbz group of 395 was subjected to deprotection using Ba(OH)2 to give 397 and 398 separable on column chromatography. The plausible intermediate formed was 396 confirmed by different experimentation. The cyclization of 397 was effected either by an earlier developed strategy using 5.0 equiv. of (n-Bu)4NBr or 10.0 equiv. of NaI in refluxing water to furnish 400 and 401 respectively along with separable compound 399. The compound 401 was found to be light sensitive and thus immediately deiodinated to Nor-11-methylpumiliotoxin 237A 402 using n-BuLi.
For the synthesis of (+)-15-(S)-pumiliotoxin A 410, opening of epoxide 392 was effected using 2.0 equiv. of alumina derivative of 403 to furnish 404. The deprotection of Cbz followed by iodide promoted cyclization produced 407 along with its epimeric congener 409. The requisite 407 was deiodinated and further reacted with Li–NH3 to afford pumilitoxin A 410 (Scheme 72).
In a similar way, the synthesis of (+)-pumiliotoxin B 415 was pursued using alkyne 411 (Scheme 72).
In a continuation of the synthetic interest, Overmann slightly modified the strategy for the diastereoselective addition of alkynes. This time the aldehyde 416 derived from proline proved to be a suitable chiral synthon for the pivotal step.121 The compound 417 prepared from L-proline120 was converted to 418 in three steps. The protection of primary –OH as SEM and subsequent hydrolysis of carbamate produced 419. The cyanomethylation of free amine and protection of secondary –OH as benzyl ether afforded 420. The deprotection of SEM group mediated by LiBF4 gave alcohol 421 which on Swern oxidation furnished the key aldehyde intermediate 416 (Scheme 73).
The diastereoselective addition of lithiated alkyne 422 to aldehyde 416 favoured the syn product 423 (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). The iodide mediated cyclization of 423 using NaI in the presence of CSA and paraformaldehyde provided 424 which on deiodination followed by debenzylation afforded 425, the nor-11-methyl analog of allopumiliotoxin 253A (Scheme 74).
1). The iodide mediated cyclization of 423 using NaI in the presence of CSA and paraformaldehyde provided 424 which on deiodination followed by debenzylation afforded 425, the nor-11-methyl analog of allopumiliotoxin 253A (Scheme 74).
For the synthesis of (+)-allopumiliotoxin 267A 428, the diastereoselective addition to the aldehyde 416 was performed using alkyne 426 to furnish 427. The similar synthetic steps as described earlier were repeated to achieve the target (Scheme 74).
In similar ways (+)-allopumiliotoxin 323B′ 429 and (+)-allopumiliotoxin 339A122 430 were synthesized by employing diastereoselective nucleophilic addition of alkynes 431 and 432 respectively upon proline derived aldehyde 416 (Scheme 75).
Tang and Montgomery conveniently utilized the intermediate 437 prepared from proline ester according to Overman's procedure,121 for the synthesis of (+)-allopumiliotoxin 267A 428, (+)-allopumiliotoxin 339B 451 and (+)-allopumiliotoxin 339A 430 using Ni catalysed silane mediated diastereoselective cyclisation (Schemes 76 and 77).123 The base hydrolysis of carbamate 437 with subsequent incorporation of the alkyne chain 438 followed by protection of the secondary –OH as benzyl ether afforded the compound 439. The compound 439 on deprotection of SEM followed by oxidation produced the requisite aldehyde 440 which on treatment with triethylsilane in the presence of Ni (COD)2 and PBu3 underwent cyclisation to produce the single isomer 441, confirmed by spectroscopic methods. The further deprotection of the protecting groups gave the target molecule (+)-allopumiliotoxin 267A 428. The synthesis of allopumiliotoxin 339B 451 and (+)-allopumiliotoxin 339A 430 were accomplished with similar synthetic sequences (Scheme 77).
Kibayashi and co-workers achieved the synthesis (+)-pumiliotoxin A 410, (+)-pumilitoxin B 415 and first total synthesis of (−)-pumiliotoxin 225F 463 by performing highly metal mediated diastereoselective nucleophilic addition of conjugated silylated compound 453 to the triflouro acetate salt 452 derived from proline (Scheme 78).124 The efficiency of the nucleophilic addition of 453 to 452 was more pronounced in the presence of Hf (92% single isomer) than Ti (71%). The transition state invoked was presumed to be metal chelated 454 which could smoothly allow the complete diastereoselectivity. The hydrostannylation of the compound 456 afforded 457 with complete trans selectivity and with higher regioselectivity along with the other minor isomer. The requisite 457 was separated and treated with NIS to give iodinated compound 458 which on subsequent carbonyl insertion produced the lactone 459.
The Boc deprotected 460 was subjected to DIBAL reduction to give 461. The cyclisation to 462 was successfully performed under Mitsunobu condition which on subsequent deprotection of terminal TBDPS group using TBAF completed the first total synthesis of pumilitoxin 225F 463. However, the optical activity observed for the synthesized compound was significantly lower than that of the natural one.
It was visualised that the intermediate 466 could be efficiently employed for the synthesis of other appendages. Thus compound 462 was transformed to iodo compound 466 through convenient synthetic sequences. After optimising the reaction conditions, a platform was set-up for the cross coupling of the iodo compound 466 with vinyl iodo compound 468 through Zn metallated halogen exchange to produce 469 by forming intermediary 467. Further classical synthetic steps accomplished the conversion of 469 to pumiliotoxin A 410. In a similar way the synthesis of pumiliotoxin B 415 was furnished by coupling the intermediate 466 with iodo compound 471.
Cossy et al. persuaded the formal synthesis of pumiliotoxin 251D 360 through chemical and photochemical induced radical cyclisation (Scheme 79).125 The strategy utilized L-proline as a starting material to construct the requisite alkyne 472 according to the literature reports.126 The Boc group of 472 was deprotected and the free NH was protected with bromopropenoyl group to afford key requisite 474. The cyclisation of 474 was carried out either by chemically or photo induced radical formation to give 475 along with the debrominated product 476. The reductive hydroxymercuration of 475 gave a mixture of epimers separable by column chromatography to give 477 which constituted the formal synthesis of pumiliotoxin 251D 360.127
Zhao and co-workers approached the formal synthesis of pumiliotoxin 251D 360 by investigating Lewis acid mediated diastereoselective nucleophilic addition of lithiated akyne to a carbonyl group (Scheme 80).128 The synthesis was processed by the preparation of tertiary alcohol 478 by Grignard addition of CH3MgI on Cbz-proline methyl ester. The reaction of the alcohol 478 with SOCl2 in Et3N afforded the alkene 417 which on ozonolysis gave the keto product 301. The metal mediated addition of ethyl lithiopropiolate to 301 gave the separable mixture of diastereomers 302a and 302b. The compound 302b was subjected to hydrogenation to produce the compound 479 to complete the formal synthesis of 360.127
Stevenson and co-workers demonstrated the study of epoxidation and dihydroxylation on indolizidine 480 for the synthesis of precursors of pumiliotoxin and allopumiliotoxin alkaloids respectively (Scheme 81).129 The strategy utilized the carbamate 162 synthesised from proline according to Overmann's procedure,124c,d converted to diene 481 which on subsequent RCM with Grubbs II catalyst produced the robust intermediate 480. The epoxidation of 480 followed by opening up of the oxirane 482 produced the single compound 483 whose structure was confirmed by X-ray analysis. The tertiary alcohol 483 on hydrogenation gave the precursor 479 for the synthesis of pumiliotoxins.127 Likewise the compound 480 was subjected to dihydroxylation followed by acetylation to isolate crude acetyl ester 484. The dihydroxylation had taken place through concave face with (R)-configuration at C-8 as anticipated. The reduction of the crude 484 with LAH and subsequent oxidation of the secondary alcohol afforded the key intermediate 485 used for the synthesis of allopumiliotoxins.130
Barrett and Damiani described a very short and concise synthesis of indolizidine 479 for the formal approach of pumiliotoxin 251 D 360 (Scheme 82).131 The synthetic strategy involved just six steps starting from Cbz-prolinal 12a. The Grignard addition of MeMgBr on 12a afforded the alcohol 486 which was as such oxidised using Jones reagent to give 301. The addition of titanium homoenolate 487 to 301 furnished the single diastereomer 488 which on subsequent hydrogenolysis of Cbz group gave the indolizidine core 479. For improvement of the yield author conveniently accessed 301 from Cbz-proline methyl ester using Tebbe reagent 489.
 | ||
| Scheme 82 Reagents and conditions: (a) MeMgBr, THF, −78 °C, 61%; (b) Jones reagent, 91%; (c) 487, −78 °C, DCM, 49%; (d) H2, Pd/C, 100%; (e) 489, 1 M HCl, acetone, 87%. | ||
The synthesis of pumiliotoxin 209F 490 and pumiliotoxin 251D 360 was described by Woodin and Jamison involves the Ni catalysed diastereoselective epoxide-alkyne reductive cyclization (Scheme 83).132 The Alloc protected proline methylate 491 (prepared from proline) was converted to keto compound 493 through the preparation of Wienreb amide 492 followed by addition of MeMgBr. The requisite epoxide 494 was synthesized by the treatment of 493 with trimethyl sulfonyl chloride in the presence of n-BuLi in high ee and de.
The effective removal of the Alloc group using catalytic Pd(dba)2 and dppb in excess diethylamine followed by treatment with the suitable alkyne 495 and 497 afforded the compounds 496 and 498 respectively. The regioselective and diastereoselective cyclization of alkyne 496 and 498 was efficiently imparted using Ni(COD)2 in the presence of trialkyl phosphines additives and Et3B furnishing the indolizidines pumiliotoxin 209F 490 and pumiliotoxin 251D 360 respectively.
Martin et al. approached the synthesis of indolizidine core 504 as a precursor for pumiliotoxin indolizidines133 via Tsuji–Trost reaction (Scheme 84).134 The commercially available L-proline was converted to Boc-proline which on DCC coupling with (OMe)NHMe·HCl afforded the Wienreb amide 499. The reaction of compound 499 with MeMgBr produced keto compound 161 which was transformed to an equimolar mixture of diastereomers 500 on treatment with ally magnesium bromide separable by standard column chromatography. The primary –OH of separated diastereomer 500b was further converted to 501 by selective protection of chiral –OH as TBS group and subsequent dihydroxylation of terminal olefin with AD-mix-α. The protection of terminal –OH as acetate and oxidation of the secondary –OH of 501 to ketone afforded the compound 502. The Wittig olefination of 502 gave the olefinic product 503 which on Boc deprotection followed by subsequent cyclisation using Tsuji–Trost reaction in the presence of Pd(PPh3)4 and Et3N furnished the indolizidine core 504.
4.6. Miscellaneous examples
Sibi and Christensen achieved the synthesis of 6-aminoindolizidine 505, an important indolizidine core present in various natural product, especially slaframine 506.92 The synthetic strategy utilized the Wittig reaction of the D-serine derived phosphorane 507 generated in situ; with Boc-prolinal 12b to afford a single isomer 508 which on subsequent hydrogenation of the double bond gave 509. The thermolytic cleavage of Boc group of 509 furnished the compound 505 (Scheme 85).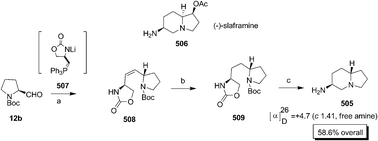 | ||
| Scheme 85 Reagents and conditions: (a) 507 (prepared in situ), THF, −78 °C, then 12b, 72%; (b) H2, Pd/C, 98%; (c) Heat, 83%. | ||
Fürstner and Kennedy efficiently synthesized some of the tylophora alkaloids through PtCl2-catalyzed cycloisomerizations and tandem deprotection-Pictet–Spengler annulations.135 The (R)-N-Boc-prolinol was subjected to 1-carbon homologation using hydroboration method and further transformed to the key alkene 511 by making use of Ohira–Bestmann reagent 510 (Scheme 86).
The alkyne 511 was subjected for coupling reaction with different iodo compounds 512, 515 and 518 to give 513, 516 and 519. These intermediates on PtCl2 catalysed cycloisomerizations followed by tandem deprotection-Pictet–Spengler cyclisation afforded the phenanthroindolizidine alakloids 514a, 517 and 520 (Scheme 87).
Ikeda et al. culminated in the total synthesis of (±)-ipalbidine 533a isolated from the seeds of Ipomoea alba L using exo-trig cyclization (Scheme 88).136 Initially the thio compound 522 synthesized from sequential homologation of Boc-prolinol was subjected to radical cyclization using Bu3SnH and AIBN in refluxing toluene, but the starting material was recovered without any notable change. The compound 521 was then transformed into selenium compound 524 and then subjected for cyclization as described earlier. A mixture of products 525 and 526 formed with the formation of anticipated 526 in low yield. For the improvement of the synthesis, the thiovinyl compound 527 was prepared and converted to thioamide 529 which underwent the expected cyclization giving the product 530 in 65% yield. The further transformation of 530 to 532 was carried out by delsulfonation and reduction. The synthesis of ipalbidine 533a was then achieved by hydrolysis of methoxy ether 532 using BBr3 but with complete racemisation.
George and Niphakis revealed the synthesis of aryl indolizidine alkaloids (+)-ipalbidine 533b and (+)-antofine 514b through endo-trig cyclization and CH activated aryl coupling reaction (Scheme 89).137 The synthesis traversed with the preparation of Wienreb amide 535 from diazo compound 534 which in turn was accessed from Boc-proline. Addition of excess ethynylmagnesiumbromide to 535 followed by subsequent Boc deprotection and neutralization furnished enaminone 536. The aromatic coupling of enaminone 536 was effetced either by iodination to give 537 followed by Buchwald's modified Suzuki–Miyaura protocol or direct Pd(OAc)2 mediated aryl coupling to furnish 539. The treatment of compound 539 with Comin's reagent gave the requisite intermediate 540 which on Negashi coupling and dimethylation gave (+)-ipalbidine 533b while Negashi reaction and subsequent treatment with hypervalent iodine reagent produced (+)-antofine 514b.
Taylor and co-workers succeeded in synthesizing grandisine B 550, which displays a potent δ-opioid receptor affinity towards human being, using L-proline as a facile starting material (Scheme 90).138 The synthesis began with the preparation of alkyne 472 from Boc-prolinal 12b using CBr4 in the presence of PPh3 using a strong base n-BuLi. The deprotection of Boc group followed by reaction with iodo acetal compound 541 afforded 542. The alkyne 542 was deprotonated and further trapped with ethyl disulfide to give 543. The compound 543 on refluxing with formic acid underwent cyclization to 544 which on subsequent desulfurization and reduction provided the alcohol precursor 545 as a pure single isomer on column purification. The compound 545 was oxidised to aldehyde 546 which was efficiently trapped using the anion of cyclohexenone 547 to give a mixture of 548a and 548b separated after column chromatography. The synthesis of grandisine B 550 was then accomplished by converting 548b to grandinisine D 549 followed by treatment with aq. NH3.
Wang and co-workers successfully achieved the first enentioselective synthesis of 13a-methyl-14-hydroxyphenanthroindolizidine alkaloids 551 and suggested the structure of hypoestestatin 2 needs to be revised further (Scheme 91).139 The requisite α-methylated proline methyl ester 554 was synthesized through Seebach's method. After several manipulations the N-alkylation of 554 with bromo compound 556 was eventually performed using K2CO3 in DCM and DMF (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), giving the product 557. The conversion of 557 to 558 was effected via Parham's type cyclisation in 70% yield. After screening the several reducing agents for the diastereoselective reduction of carbonyl, Et3BH was found to be the best in discriminating the isomers giving the separable mixture of 551c and 551d. In a similar way the other two possible isomers 551a and 551b were synthesized using ent-554. The detail optical activity study and NMR revealed that the physical data of none of the isomers synthesized matching with that reported for hypoestestatin 2 showcased the need of further study in this field.
1), giving the product 557. The conversion of 557 to 558 was effected via Parham's type cyclisation in 70% yield. After screening the several reducing agents for the diastereoselective reduction of carbonyl, Et3BH was found to be the best in discriminating the isomers giving the separable mixture of 551c and 551d. In a similar way the other two possible isomers 551a and 551b were synthesized using ent-554. The detail optical activity study and NMR revealed that the physical data of none of the isomers synthesized matching with that reported for hypoestestatin 2 showcased the need of further study in this field.
Very recently (S)-(+)-tylophorine 520 was synthesized by Stoye and Opatz through free-radical cyclization of an N-aziridinylimine (Scheme 92).140 The starting dibromo compound 561 was prepared by condensation of homoveratric acid and veratraldehyde followed by oxidative cycization and bromination. The N alkylation of methyl proline ester with 561 gave 562 which on DIBAL reduction of the ester followed by subsequent condensation with amino aziridine 563 afforded 564. The cyclisation of 564 was effected using Ph3SnH in the presence of AIBN to furnish the natural product 520.
5. Summary
The present review explicitly describes the versatility of proline particularly emphasizing it as a unique chiral synthon for the synthesis of naturally occurring pyrrolidines, pyrrolizidines and indolizidine alkaloids. The synthesis of a wide spectrum of natural products has been derived ranging from simple to complex molecules, that has placed proline on a cutting edge in chiral pool synthesis. The construction of various heteroatom-impregnated cyclic compounds are deemed to be useful for synthetic chemists for further tuning of these strategies. It is worth mentioning that manifolds of proline-derived heterocyclic scaffolds, similar to those natural products described in this review, have been synthesized and undoubtedly have identified proline as a robust “chiral tool” in the pharmaceuticals and biotechnological fields. The review has also rationalized the synthesis of bulky alkaloids like dolastatin and pumiliotoxins using proline as a synthetic precursor which will be useful for synthetic chemists working specifically to design novel protocols for the improvement of syntheses. The collection also provides room for synthetic chemists to alleviate or obviate indigenous racemisation of the intermediates and final natural products caused by several reagents and reaction conditions during synthetic manipulation. More specifically, the present report has established proline as a competent and leading amino acid for the synthesis of asymmetric natural products besides its ‘universal application’ as an organocatalyst. The vast coverage of the syntheses that has taken place between 1990 and the present day will help readers to comprehend most of the proline based chiral pool synthesis of the aforementioned alkaloids, since very few syntheses have previously been reported.Acknowledgements
The author, C. Bhat is thankful to CSIR, New Delhi, for awarding CSIR-NET-SRF. We are also grateful to DST, New Delhi, for financial assistance.References and notes
-
(a) S. Hanessian, Total Synthesis of Natural Products: the ‘Chiron’ Approach, ed. J. E. Baldwin, Pergamon, Oxford, UK, 1983 Search PubMed
; (b) W. A. Nugent, T. V. Rajanbabu and M. J. Burk, Science, 1993, 259, 479 CAS
.
- M. S. Taylor and E. N. Jacobsen, Proc. Natl. Acad. Sci. U. S. A., 2004, 101, 5368 CrossRef CAS PubMed
.
- Amino acids, Peptides and Proteins in Organic Chemistry, ed. A. B. Hughes, Wiley-VCH, Weinheim, 2009 Search PubMed
.
- D. S. Verma and A. J. Delauney, Plant J., 1993, 4, 215 Search PubMed
.
- For recent reviews on proline as an organocatalyst see:
(a) S. K. Panday, Tetrahedron: Asymmetry, 2011, 22, 1817 CrossRef CAS PubMed
; (b) B. List, Tetrahedron, 2002, 58, 5573 CrossRef CAS
.
-
(a) M. Naser, P. Khalil, K. Shabnaz and R. B. Montazer, Asian J. Chem., 2012, 24, 4791 Search PubMed
; (b) N. S. Zeba and F. Farheen, Catal. Sci. Technol., 2011, 5, 134 Search PubMed
; (c) N. S. Zeba and M. T. N. Mohammed, Tetrahedron Lett., 2011, 52, 4008 CrossRef PubMed
; (d) R. Fernandez-Lopez, J. Kofoed, M. Machuqueiro and T. Drabre, Eur. J. Org. Chem., 2005, 5268 CrossRef CAS
.
-
(a) J. P. Michael, Nat. Prod. Rep., 2005, 22, 603 RSC
; (b) Y. Cheng, Z. T. Huang and M. X. Wang, Curr. Org. Chem., 2004, 8, 325 CrossRef CAS
.
-
(a) C. Alvarez-Ibarra, A. G. Csaky, G. A. Lopez de Silanes and M. L. Quiroga, J. Org. Chem., 1997, 62, 479 CrossRef CAS PubMed
; (b) A. Bianco, M. Maggini, G. Scorrano, C. Toniolo, G. Marconi, C. Villani and M. Prato, J. Am. Chem. Soc., 1996, 118, 4072 CrossRef CAS
.
-
(a) J. Seayad and B. List, Org. Biomol. Chem., 2005, 3, 719 RSC
; (b) P. I. Dalko and L. Moisan, Angew. Chem., Int. Ed., 2004, 43, 5138 CrossRef CAS PubMed
.
-
(a) W. Notz, F. Tanaka and C. F. Barbas, Acc. Chem. Res., 2004, 37, 580 CrossRef CAS PubMed
; (b) F. X. Felpin and J. Lebreton, Eur. J. Org. Chem., 2003, 3693 CrossRef CAS
; (c) W. H. Pearson and P. Stoy, Synlett, 2003, 903 CrossRef CAS
; (d) W. H. Pearson, Pure Appl. Chem., 2002, 74, 1339 CrossRef CAS
.
-
(a) G. Cignarella, C. G. Gallo and E. Testa, J. Am. Chem. Soc., 1961, 83, 4999 CrossRef CAS
; (b) R. Noyori, Y. Baba and H. Hayakawa, J. Am. Chem. Soc., 1974, 96, 3336 CrossRef CAS
; (c) G. Forder and R. Dharanipragada, Nat. Prod. Rep., 1993, 199 Search PubMed
; (d) M. Majewski and R. Lazny, J. Org. Chem., 1995, 60, 5825 CrossRef CAS
; (e) M. Jordan, M. Human, S. Bieri, C. Christen, E. Poblete and O. Munoz, Phytochemistry, 2006, 67, 570 CrossRef CAS PubMed
; (f) T. Yamauchi, S. Hagiwara and K. Higashiyama, J. Org. Chem., 2008, 73, 9784 CrossRef CAS PubMed
; (g) G. M. Sandala, D. M. Smith and L. Radom, J. Am. Chem. Soc., 2008, 130, 10684 CrossRef CAS PubMed
; (h) F. A. Davis, N. Theddu and P. M. Gaspari, Org. Lett., 2009, 11, 1647 CrossRef CAS PubMed
.
- T. Shono, Y. Matsumura, K. Tsubata and K. Uchida, J. Org. Chem., 1986, 51, 2590 CrossRef CAS
.
-
(a) I. Komuis, W. Mueller, E. Boehni, R. Then and M. Montavan, Eur. J. Med. Chem., 1977, 12, 536 Search PubMed
; (b) H. Iida, S. Aoyagi, K. Kawano and C. Kibayashi, Chem. Pharm. Bull., 1978, 26, 3229 CrossRef CAS
; (c) For a review on enamino ketones, see: J. V. Greenhill, Chem. Soc. Rev., 1977, 6, 277 RSC
.
- E. B. Arévalo-García and J. C. Q. Colmenares, Tetrahedron Lett., 2008, 49, 3995 CrossRef PubMed
.
- S. G. Tilve and M. S. Majik, Tetrahedron Lett., 2010, 51, 2900 CrossRef PubMed
.
- C. Bhat and S. G. Tilve, Tetrahedron Lett., 2011, 52, 6566 CrossRef CAS PubMed
.
- C. E. Turner, M. A. Elsohly, L. Hanus and H. N. Elsohly, Phytochemistry, 1981, 20, 1403 CrossRef CAS
.
- Y. Jagadeesh, K. K. S. Reddy and B. V. Rao, Tetrahedron: Asymmetry, 2011, 22, 1485 CrossRef CAS PubMed
.
- G. R. Pettit, Y. Kamano, C. L. Herald, A. A. Tuinman, F. E. Boettner, H. Kizu, J. M. Schmidt, L. Baczynskyj, K. B. Tomer and R. J. Bontems, J. Am. Chem. Soc., 1987, 109, 6883 CrossRef CAS
.
- G. R. Pettit, S. B. Singh, F. Hogan, P. Lloyd-Williams, D. D. Burkett and P. J. Clewlow, J. Am. Chem. Soc., 1989, 111, 5463 CrossRef CAS
.
- T. Madden, H. T. Tran, D. Beck, R. Huie, R. A. Newman, L. Pusztai, J. J. Wright and J. L. Abbruzzese, Clin. Cancer Res., 2000, 6, 1293 CAS
.
- G. R. Petit, S. B. Singh, D. L. Herald, P. Lloyd-Williams, D. Kantoci, D. D. Burkett, J. Barkóczy, F. Hogan and T. R. Wardlaw, J. Org. Chem., 1994, 59, 6287 CrossRef
.
- G. R. Petit, D. D. Burkett, J. Barkóczy, G. L. Breneman and W. E. Petit, Synthesis, 1996, 719 CrossRef PubMed
.
-
(a) T. Shioiri, K. Hayashi and Y. Hamada, Tetrahedron Lett., 1991, 32, 931 CrossRef
; (b) T. Shioiri, K. Hayashi and Y. Hamada, Tetrahedron, 1993, 49, 1913 CrossRef CAS
.
- G. R. Petit and M. P. Grealish, J. Org. Chem., 2001, 66, 8640 CrossRef
.
- W. P. Almeida and F. Coelho, Tetrahedron Lett., 2003, 44, 937 CrossRef CAS
.
-
(a) D. Lavergne, C. Mordant, V. Ratovelomanana-Vidal and J.-P. Genet, Org. Lett., 2001, 3, 1909 CrossRef CAS PubMed
; (b) C. Mordant, S. Reymond, V. Ratovelomanana-Vidal and J.-P. Genet, Tetrahedron, 2004, 60, 9715 CrossRef CAS PubMed
; (c) C. Mordant, S. Reymond, H. Tone, D. Lavergne, R. Touati, B. B. Hassine, V. Ratovelomanana-Vidal and J.-P. Genet, Tetrahedron, 2007, 63, 6115 CrossRef CAS PubMed
.
- R. Cella, R. C. Venturoso and H. A. Stefani, Tetrahedron Lett., 2008, 49, 16 CrossRef CAS PubMed
.
- F. Roux, I. Maugras, J. Poncet and G. N. P. Jouin, Tetrahedron, 1994, 50, 5345 CrossRef CAS
.
- K. Tomioka, M. Kanai and K. Koga, Tetrahedron Lett., 1991, 32, 2395 CrossRef CAS
.
- K. C. Woo and K. Jones, Tetrahedron, 1991, 47, 7179 CrossRef
.
- F. Roessler, D. Ganzmger, S. Johne, E. Schopp and M. Hesse, Helv. Chim. Acta, 1978, 61, 1200 CrossRef CAS
.
- X. Shi, A. B. Attygalle, S. C. Xu, V. U. Ahmed and J. Meinwald, Tetrahedron, 1996, 52, 6859 CrossRef CAS
.
- A. B. Attygalle, S.-C. Xu, K. D. McCormick, J. Meinwald, C. L. Blankespoor and T. Eisner, Tetrahedron, 1993, 49, 9333 CrossRef CAS
.
- D. Enders and C. Thiebes, Pure Appl. Chem., 2001, 73, 573 CrossRef CAS
.
- D. Enders and C. Thiebes, Synthesis, 2000, 510 CrossRef CAS PubMed
.
- P. Blanco, F. Busqué, P. March, M. Figueredo, J. Font and E. Sanfeliu, Eur. J. Org. Chem., 2004, 48 CrossRef CAS
.
- T. Sato, K. Tsujimoto, K. Matsubayashi, H. Ishibashi and M. Ikeda, Chem. Pharm. Bull., 1992, 40, 2308 CrossRef CAS
.
- H. Takayama, R. Sudo and M. Kitajima, Tetrahedron Lett., 2005, 46, 5795 CrossRef CAS PubMed
.
- E. J. Corey, S. Shibata and R. K. Bakshi, J. Org. Chem., 1988, 53, 2861 CrossRef CAS
.
- M. A. Tan, M. Kitajima, N. Kogure, M. G. Nonato and H. Takayama, J. Nat. Prod., 2010, 73, 1453 CrossRef CAS PubMed
.
- L. Hu, L. Zhang and H. Zhai, J. Org. Chem., 2009, 74, 7552 CrossRef CAS PubMed
.
- J. Kubanek, D. E. Williams, E. D. Silva, T. Allen and R. J. Andersen, Tetrahedron Lett., 1995, 36, 6189 CrossRef CAS
.
- R. Grote, A. Zeeck, J. Stuempfel and H. Zaehner, Liebigs Ann. Chem., 1990, 6, 525 CrossRef
.
- M. S. Majik, P. S. Parameswaran and S. G. Tilve, J. Chem. Res., 2008, 3, 121 CrossRef
.
- S. Arlette, A. Khalid, S. Michel and W. Richard, Tetrahedron: Asymmetry, 2005, 16, 1055 CrossRef PubMed
.
- A. M. Fournier, R. A. Brown, W. Farnaby, H. Miyatake-Ondozabal and J. Clayden, Org. Lett., 2010, 12, 2222 CrossRef CAS PubMed
.
- H. Konno, S. Kusumoto, S. Kanai, Y. Yamahana, K. Nosaka and K. Akaji, Heterocycles, 2006, 68, 2579 CrossRef CAS PubMed
.
- L. Yang, Y. Wang, Z. M. Bi, P. Lin, Z. T. Wang and L. S. Xu, Chin. J. Nat. Med., 2004, 2, 280 CAS
and the references cited there in.
-
(a) J. R. Liddell, Nat. Prod. Rep., 2001, 18, 441 RSC
; (b) J. B. Harborne, Nat. Prod. Rep., 2001, 18, 361 RSC
; (c) J. R. Trigo, J. Braz. Chem. Soc., 2000, 11, 551 CrossRef CAS PubMed
; (d) A. M. Rizk, Naturally Occurring Pyrrolizidine Alkaloids, CRC Press, Boca raton, 1991 Search PubMed
.
-
(a) P. P. Fu, Q. Xia, G. Lin and M. W. Chou, Drug Metab. Rev., 2004, 36, 1 CrossRef CAS PubMed
; (b) M. T. Chakler, Hepatology, 2003, 39, 437 Search PubMed
; (c) B. L. Stegelmeier, J. A. Edgar, S. M. Colegate, D. R. Gardner, T. K. Schoch, R. A. Coulombe and R. T. Molyneux, J. Nat. Toxins, 1999, 8, 95 CAS
; (d) E. Rodel, Pharmazie, 2000, 55, 711 Search PubMed
; (e) A. R. Mattock, The Chemistry and Toxicology of Pyrrolizidine Alkaloids, Academic Press, London, 1986 Search PubMed
; (f) C. J. Culvenor, Trends Pharmacol. Sci., 1985, 6, 18 CrossRef CAS
; (g) L. W. Smith and C. J. Culvenor, J. Nat. Prod., 1981, 44, 129 CrossRef CAS
; (h) A. R. Mattocks and L. N. H. White, Chem.-Biol. Interact., 1976, 15, 173 CrossRef CAS
.
-
(a) P. Compain and O. R. Martin, Bioorg. Med. Chem., 2001, 9, 3077 CrossRef CAS
; (b) A. Watson, G. W. J. Fleet, N. Asano, R. J. Molyneux and R. J. Nash, Phytochemsitry, 2001, 56, 265 CrossRef CAS
; (c) N. Asano, A. Kato and A. A. Watson, Mini-Rev. Med. Chem., 2001, 1, 145 CrossRef CAS
; (d) N. Asano, A. R. Nash, R. J. Molyneux and G. W. J. Fleet, Tetrahedron: Asymmetry, 2000, 11, 1645 CrossRef CAS
.
- R. Grote, A. Zeeck, J. Stümpfel and H. Zähner, Liebigs Ann. Chem., 1990, 525 CrossRef CAS
.
- A. R. Mattock, Chemistry and Toxicology of Pyrrolizidine Alkaloids, Academic Press, London, 1986 Search PubMed
.
- Y. Aoyagi, T. Manabe, T. Ohta, T. Kurihara, G.-L. Pang and T. Yuhara, Tetrahedron, 1996, 52, 869 CrossRef CAS
.
- G. B. Giovenzana, M. Sisti and G. Palmisano, Tetrahedron: Asymmetry, 1997, 8, 515 CrossRef CAS
.
- M. Arisawa, M. Takahashi, E. Takezawa, T. Yamaguchi, Y. A. Torisawa and M. Nakagawa, Chem. Pharm. Bull., 2000, 48, 1593 CrossRef CAS
.
-
(a) M. S. Majik, S. Jyoti, S. G. Tilve and P. S. Parameswaran, Synthesis, 2007, 663 CAS
; (b) M. S. Majik, P. S. Parameswaran and S. G. Tilve, Helv. Chim. Acta, 2008, 91, 1500 CrossRef CAS
.
- A. Murray, G. R. Proctor and P. J. Murray, Tetrahedron Lett., 1995, 36, 291 CrossRef CAS
.
- R. Schobert and A. Wicklein, Synthesis, 2007, 1499 CrossRef CAS PubMed
.
- J. G. Knight and S. V. Ley, Tetrahedron Lett., 1991, 32, 7119 CrossRef CAS
.
- H. Ishibashi, N. Uemura, H. Nakatani, M. Okazaki, T. Sato, N. Nakamura and M. Ikeda, J. Org. Chem., 1993, 58, 2360 CrossRef CAS
.
- T. Sato, K. Taujimoto, K. Matsubayashi, H. Ishibashi and M. Ikeda, Chem. Pharm. Bull., 1992, 40, 2308 CrossRef CAS
.
- J. A. Seijas, M. P. Vázquez-Tato, L. Castedo, R. J. Estévez, M. G. Ónega and M. Ruíz, Tetrahedron, 1992, 48, 1637 CrossRef CAS
.
- M. Mori, N. Kanda, I. Oda and Y. Ban, Tetrahedron, 1985, 41, 5465 CrossRef CAS
.
- M. Taddei, F. Thareau, A. Mann and S. Coz, Heterocycles, 1993, 36, 2073 CrossRef PubMed
.
- P. H. J. Carlsen, T. Katsuki, V. S. Martin and K. B. Sharpless, J. Org. Chem., 1981, 46, 3936 CrossRef CAS
.
- A. Hassner, S. Singh, R. Sharma and R. Maurya, Tetrahedron, 1993, 49, 2317 CrossRef CAS
.
- A. Hassner, R. Maurya, A. Padwa and W. H. Bullock, J. Org. Chem., 1991, 56, 2775 CrossRef CAS
.
- A. Murray, G. R. Proctor and P. J. Murray, Tetrahedron, 1996, 52, 3757 CrossRef CAS
.
- H. Ishibashi, M. Sasaki and T. Taniguchi, Tetrahedron, 2008, 64, 7771 CrossRef CAS PubMed
.
- K. K. S. Reddy, B. V. Rao and S. S. Raju, Tetrahedron: Asymmetry, 2011, 22, 662 CrossRef CAS PubMed
.
- M.-Y. Chang, D.-C. Wu and N.-C. Chang, Heterocycles, 2005, 65, 2965 CrossRef CAS PubMed
and references cited therein.
- D. Craig, C. J. T. Hyland and S. E. Ward, Synlett, 2006, 2142 CrossRef CAS PubMed
.
- I. L. Lysenko and O. G. Kulinkovich, Russ. J. Org. Chem., 2005, 41, 73 CrossRef PubMed
.
- D. W. Knight, A. C. Share and P. T. Gallagher, J. Chem. Soc., Perkin Trans. 1, 1997, 2089 RSC
.
- J. Mulzer and M. Shanyoor, Tetrahedron Lett., 1993, 34, 6545 CrossRef CAS
.
- T. Moriwake, S. Hamano, D. Miki, S. Saito and S. Torii, Chem. Lett., 1986, 815 CrossRef CAS
.
- P. Guerreiro, V. Ratovelomanana-Vidal and J.-P. Genét, Chirality, 2000, 12, 408 CrossRef CAS
.
-
(a) Patent: Daiichi Seiyaku Co, Ltd., Derivatives of penem, EP 0210883, June 1986
; (b) D. W. Brooks, L. D.-L. Lu and S. Masamune, C-alkylation under neutral conditions, Angew. Chem., Int. Ed., 1979, 18, 72 CrossRef
.
- I. Izquierdo, M. T. Plaza and V. Yáňez, Tetrahedron: Asymmetry, 2005, 16, 3887 CrossRef CAS PubMed
.
- H. Ito, Y. Ikeuchi, T. Taguchi and Y. Hanzawa, J. Am. Chem. Soc., 1994, 116, 5469 CrossRef CAS
.
- M. Daurte, G. Stedele, M. Pazinatto, E. R. de Oliveira and V. L. Eifler-Lima, Lett. Org. Chem., 2009, 6, 90 CrossRef
.
- E. J. Eklund, R. D. Pike and J. R. Scheerer, Tetrahedron Lett., 2012, 53, 4644 CrossRef CAS PubMed
.
- S. G. Davies, A. M. Fletcher, P. M. Roberts and J. E. Thomson, Tetrahedron: Asymmetry, 2012, 23, 1111 CrossRef CAS PubMed
.
- S. G. Davies, A. M. Fletcher, C. Lebée, P. M. Roberts, J. E. Thomson and J. Yin, Tetrahedron, 2012, 69, 1369 CrossRef PubMed
.
- C. Christine, K. Ikhiri, A. Ahond, A. A. Mourabit, C. Poupat and et P. Potier, Tetrahedron, 2000, 56, 1837 CrossRef CAS
.
-
(a) J. P. Michael, Nat. Prod. Rep., 2007, 24, 191 RSC
; (b) J. W. Daly, H. M. Garraffo and T. F. Spande, J. Nat. Prod., 2005, 68, 1556 CrossRef CAS PubMed
; (c) J. W. Daly, J. Med. Chem., 2003, 46, 445 CrossRef CAS
; (d) J. R. Lewis, Nat. Prod. Rep., 2001, 18, 95 RSC
; (e) D. O. Hagan, Nat. Prod. Rep., 2000, 17, 435 RSC
.
-
(a) L. E. Fellows and G. W. J. Fleet, in Natural Products Isolation, ed. G. H. Wafman and R. Cooper, Elsevier, Amsterdam, 1989, p. 539 Search PubMed
; (b) H. Takahata and T. Momose, in The Alkaloids, ed. G. A. Cordell, Academic, San Diego, CA, 1993, vol. 44, ch. 3 Search PubMed
; (c) J. P. Michael, Nat. Prod. Rep., 1999, 16, 675 RSC
.
- J. P. Michael and D. Gravestock, Eur. J. Org. Chem., 1998, 865 CrossRef CAS
and references cited therein.
-
(a) J. W. Daly, H. M. Garraffo, T. F. Spande, in Alkaloids: Chemical and Biological Perspectives, ed. Pelletier, S. W., Pergamon Press, Amsterdam, 1999, vol. 13, pp. 1–161 Search PubMed
; (b) General reviews on indolizidine alkaloids: J. P. Michael, Nat. Prod. Rep., 1997, 14, 21 RSC
; (c) H. Takahata, T. Momose, in The Alkaloids, ed. G. A. Cordell, Academic, San Diego, 1993, vol. 44, ch. 3 Search PubMed
.
- M. P. Sibi and J. W. Christensen, J. Org. Chem., 1999, 64, 6434 CrossRef CAS
.
- S. H. Park, H. J. Kang, S. Ko, S. Park and S. Chang, Tetrahedron: Asymmetry, 2001, 12, 2621 CrossRef CAS
.
- S. Nukui, M. Sodeoka and M. Shibasaki, Tetrahedron Lett., 1993, 34, 4965 CrossRef CAS
.
- V. D. Pinho and A. C. B. Burtoloso, Tetrahedron Lett., 2012, 53, 876 CrossRef CAS PubMed
.
- G. Lhommet, H. Dhimane and C. Céliméne, Tetrahedron, 1998, 54, 10457 CrossRef
.
-
(a) T. Shono, Y. Matsumura and K. Tsubata, Org. Synth., 1985, 63, 206 CAS
; (b) T. Shono, Y. Matsumura, T. Kanazawa, M. Habuka, K. Unchida and K. Toyoda, J. Chem. Res., Synop., 1984, 320 (J. Chem. Res., Miniprint, 1984, 2876) CAS
.
- Z. Gang, Z. Yazhu, Y. Menglong and W. Hao, Chin. J. Chem., 2009, 27, 183 CrossRef
.
- T. G. Back and K. Nakajima, J. Org. Chem., 2000, 65, 4543 CrossRef CAS PubMed
.
- T. Ponpandian and S. Muthusubramanian, Tetrahedron, 2013, 9, 527 CrossRef PubMed
.
- T.-H. Chan and Y. St-Denis, J. Org. Chem., 1992, 57, 3078 CrossRef
.
- H. Zhang, Y.-K. Ni, G. Zhao and Y. Ding, Eur. J. Org. Chem., 2003, 1918 CrossRef CAS
.
- A. M. P. Koskinen and O. A. Kallatsa, Tetrahedron, 2003, 59, 6947 CrossRef CAS
.
- C. H. Heathcock and T. W. von Geldern, Heterocycles, 1987, 25, 75 CrossRef CAS PubMed
.
- P. R. Sultane, A. R. Mohite and R. G. Bhat, Tetrahedron Lett., 2012, 53, 5856 CrossRef CAS PubMed
.
- B. Bernardim, V. D. Pinho and A. C. B. Burtoloso, J. Org. Chem., 2012, 77, 9926 CrossRef CAS PubMed
.
-
(a) A. Sudau, W. Münch, J.-W. Bats and U. Nubbemeyer, Eur. J. Org. Chem., 2002, 3304 CrossRef CAS
; (b) A. Sudau, W. Münch, J.-W. Bats and U. Nubbemeyer, Eur. J. Org. Chem., 2002, 3315 CrossRef CAS
.
-
(a) A. G. H. Wee, G.-J. Fan and H. M. Bayirinoba, J. Org. Chem., 2009, 74, 8261 CrossRef CAS PubMed
; (b) H. Zhang, X. Li, H. Huang and P. Huang, Sci. China Chem., 2011, 54, 737 CrossRef CAS
; (c) H. K. Lee, J. S. Chun and C. S. Pak, J. Org. Chem., 2003, 68, 2471 CrossRef CAS PubMed
.
- H. Yun, J. Kim, J. Sim, S. Lee, Y. T. Han, D.-J. Chang, D.-D. Kim and Y.-G. Suh, J. Org. Chem., 2012, 77, 5389 CrossRef CAS PubMed
.
- J. Cossy, D. G. Pardo and I. Déchamps, Tetrahedron, 2007, 63, 9082 CrossRef PubMed
.
- R. C. Fuson and C. L. Zirkle, J. Am. Chem. Soc., 1948, 70, 2760 CrossRef CAS
.
- S. J. Oxenford, S. P. Moore, G. Carbone, G. Barker, P. O'Brien, M. R. Shipton, J. Gilday and K. R. Campos, Tetrahedron: Asymmetry, 2010, 21, 1563 CrossRef CAS PubMed
.
-
(a) B. P. Bashyal, G. W. J. Fleet, M. J. Gough and P. W. Smith, Tetrahedron, 1987, 43, 3083 CrossRef CAS
; (b) R. B. Bennett, J. R. Choi, W. D. Montgomery and J. K. Cha, J. Am. Chem. Soc., 1989, 111, 2580 CrossRef CAS
; (c) W. H. Pearson, Y. Ren and J. D. Powers, Heterocycles, 2002, 58, 421 CrossRef CAS PubMed
.
- N. Buschmann, A. Rückert and S. Bletchert, J. Org. Chem., 2002, 67, 4325 CrossRef CAS PubMed
.
-
(a) J. W. Daly and C. W. Myers, Science, 1967, 156, 970 CAS
; (b) For a review of pumiliotoxins, see: J. W. Daly, T. F. Spande and H. M. Garraffo, J. Nat. Prod., 2005, 68, 1556 CrossRef CAS PubMed
.
-
(a) M. Endoh, Br. J. Pharmacol., 2004, 143, 663 CrossRef CAS PubMed
; (b) E. X. Albuquerque, J. E. Warnick, M. A. Maleque, F. C. Kauffman, R. Tamburini, Y. Nimit and J. W. Daly, Mol. Pharmacol., 1981, 19, 411 CAS
; (c) F. Gusovsky, W. L. Padgett, C. R. Creveling and J. W. Daly, Mol. Pharmacol., 1992, 42, 1104 CAS
.
-
(a) S. Inoue and K. Honda, J. Synth. Org. Chem., Jpn., 1993, 51, 894 CrossRef CAS
; (b) R. C. Larock, in Advances in Metal-Organic Chemistry, JAI, Greenwich, 1994, vol. 3, pp. 97–224 Search PubMed
; (c) W. R. Ashcroft and J. A. Joule, Tetrahedron Lett., 1980, 21, 2341 CrossRef CAS
; (d) K. C. Nicolaou, D. A. Claremon and W. E. Barnette, J. Am. Chem. Soc., 1980, 102, 6611 CrossRef CAS
.
- A. S. Franklin and L. E. Overmann, Chem. Rev., 1996, 96, 505 CrossRef CAS PubMed
.
- N.-H. Lin, L. E. Overman, M. H. Rabinowitz, L. A. Robinson, M. J. Sharp and J. Zablocki, J. Am. Chem. Soc., 1996, 118, 9062 CrossRef CAS
.
-
(a) A. S. Franklin and L. E. Overman, Chem. Rev., 1996, 96, 505 CrossRef CAS PubMed
; (b) N. H. Lin, L. E. Overman, M. H. Rabinowitz, L. A. Robinson, M. J. Sharp and J. Zablocki, J. Am. Chem. Soc., 1996, 118, 9062 CrossRef CAS
.
- C. Caderas, R. Lett, L. E. Overman, M. H. Rabinowitz, L. A. Robinson, M. J. Sharp and J. Zablocki, J. Am. Chem. Soc., 1996, 118, 9073 CrossRef CAS
.
- L. E. Overman, L. A. Robinson and J. Zablocki, J. Am. Chem. Soc., 1992, 114, 368 CrossRef CAS
.
- J. Montgomery and X.-Q. Tang, J. Am. Chem. Soc., 1999, 121, 6098 CrossRef
.
-
(a) S. Hirashima, S. Aoyagi and C. Kibayashi, J. Am. Chem. Soc., 1999, 121, 9873 CrossRef CAS
; (b) S. Aoyagi, S. Hirashima, K. Saito and C. Kibayashi, J. Org. Chem., 2002, 67, 5517 CrossRef CAS PubMed
; (c) B. M. Trost and T. S. Scanlan, J. Am. Chem. Soc., 1989, 111, 4988 CrossRef CAS
; (d) S. W. Goldstein, L. E. Overman and M. H. Rabinowitz, J. Org. Chem., 1992, 57, 1179 CrossRef CAS
.
- J. Cossy, M. Cases and D. G. Pardo, Synlett, 1996, 909 CrossRef CAS PubMed
.
- J. Y.-L. Chung and J. T. Wasicak, Tetrahedron Lett., 1990, 31, 3957 CrossRef CAS
.
- D. N. A. Fox, D. Lathbury, M. F. Mahon, K. C. Molloy and T. Gallagher, J. Am. Chem. Soc., 1991, 113, 2652 CrossRef CAS
.
- Y. Ni, G. Zhao and Y. Ding, J. Chem. Soc., Perkin Trans. 1, 2000, 3264 RSC
.
- G. O'Mahony, M. Nieuwenhuyzen, P. Armstrong and P. J. Stevenson, J. Org. Chem., 2004, 69, 3968 CrossRef CAS PubMed
.
-
(a) L. E. Overman and S. W. Goldstein, J. Am. Chem. Soc., 1984, 106, 5360 CrossRef CAS
; (b) S. W. Goldstein, L. E. Overman and M. H. Rabinowitz, J. Org. Chem., 1992, 57, 1179 CrossRef CAS
.
- A. G. M. Barrett and F. Damiani, J. Org. Chem., 1999, 64, 1410 CrossRef CAS
.
- K. S. Woodin and T. F. Jamison, J. Org. Chem., 2007, 72, 7451 CrossRef CAS PubMed
.
- L. E. Overman and D. Lesuisse, Tetrahedron Lett., 1985, 26, 4167 CrossRef CAS
.
- R. E. Martin, M. E. Polomska, L. T. Byrne and S. G. Stewart, Tetrahedron Lett., 2011, 52, 4878 CAS
.
- A. Fürstner and W. J. Kennedy, Chem.–Eur. J., 2006, 12, 7398 CrossRef PubMed
.
- M. Ikeda, J. Shikaura, N. Maekawa, K. Daibuzono, H. Teranishi, Y. Teraoka, N. Oda and H. Ishibashi, Heterocycles, 1999, 50, 31 CrossRef CAS PubMed
.
- M. J. Niphakis and G. I. Georg, J. Org. Chem., 2010, 75, 6019 CrossRef CAS PubMed
.
- J. D. Cuthbertson, A. A. Godfrey and R. J. K. Taylor, Org. Lett., 2011, 13, 3976 CrossRef CAS PubMed
.
- B. Su, M. Deng and Q. Wang, Eur. J. Org. Chem., 2013, 1979 CrossRef CAS
.
- A. Stoye and T. Opatz, Org. Lett., 2010, 12, 2140 CrossRef CAS PubMed
.
| This journal is © The Royal Society of Chemistry 2014 |