Osteogenesis of human induced pluripotent stem cells derived mesenchymal stem cells on hydroxyapatite contained nanofibers†
Ran Kangad,
Yonglun Luo*c,
Lijin Zoua,
Lin Xied,
Helle Lysdahla,
Xiumei Jiange,
Chunying Chene,
Lars Bolundc,
Menglin Chen*b,
Flemming Besenbacherb and
Cody Büngera
aOrthopaedic Research Lab, Aarhus University Hospital, Aarhus C 8000, Denmark
bInterdisciplinary Nanoscience Center, Aarhus University, Aarhus C 8000, Denmark. E-mail: menglin@inano.au.dk
cDepartment of Biomedicine, Aarhus University, Aarhus C 8000, Denmark. E-mail: alun@hum-gen.au.dk
dJiangsu Province Hospital on Integration of Chinese and Western Medicine, Nanjing 210028, China
eCAS Key Laboratory for Biomedical Effects of Nanomaterials and Nanosafety, National Center for Nanoscience and Technology of China, Beijing 100190, China
First published on 17th December 2013
Abstract
Biomimetic nanofibrous scaffolds combined with stem cells are promising for bone tissue engineering. In the present study, we have employed nano-hydroxyapatite (nHAp) contained polycaprolactone (PCL) nanofibers as a biomimetic nanofibrous scaffold, and mesenchymal stem cells derived from human induced pluripotent stem cells (hiPS-MSCs) as the novel stem cells sources. The response of hiPS-MSCs on the nanofibrous scaffolds in terms of cell proliferation and differentiation into the osteoblastic phenotype was investigated by XTT assay, scanning electron microscopy (SEM), osteogenic genes expression (runt-related transcription factor 2 (RUNX2), alkaline phosphatase (ALP), collagen I (COL1A1), and osteocalcin (OC)), ALP activity, and calcium deposition. It is clearly shown that the hiPS-MSCs attached, and proliferated on the nanofibrous scaffolds. Compared with PCL nanofibers without nHAp, the cells on the nHAp contained nanofibers demonstrated superior capabilites to differentiate to form calcified extracellular matrix. Together with gene expression, all of the results indicate the great potential of the hiPS-MSCs seeded biomimetic nanofibrous scaffolds for bone regeneration in the future.
Introduction
Mimicking cellular micro-environments and niches using biocompatible polymers will facilitate stem cells proliferation and differentiation for tissue regeneration.1,2 The significance of the overall fibrillar and porous nanoscale topography of the extracellular matrix (ECM) in promoting essential cellular processes has led tissue engineers to replace macroporous scaffolds to biomimetic materials with nanofibrous features. A unique technique that has gained tremendous attention in the last decade as the most robust, straightforward nanofibre processing method is electrospinning.3 The technique becomes extremely intriguing as it allows straightforward incorporation of bioactive ingredients in one-step co-spinning process.3 Thus, biological active signals, such as ECM components or growth factors, could be easily incorporated into these fibers giving them the potency to induce, enhance the cell growth, differentiation, and achieve in more effective tissue engineering.4,5 Hydroxyapatite (HAp) is chemically similar to the mineral component of bones in mammals. Due to their bone bioactive potential (e.g., osteoinductivity, osteoconductivity and osteointegration), HAp are widely employed to facilitate stem-cell osteogenic differentiation.6,7 Nano-sized HAp (nHAp), which better mimics the HAp crystals in natural tissues, is more promising for bone tissue engineering.8,9Mesenchymal stem cells (MSCs) are commonly used for bone tissue engineering because of their unique biological property of differentiation into mesodermal derivatives10,11 and also their immunomodulatory and engraftment-promoting properties.12–14 Therefore, the interactivity of MSCs and nanofibrous scaffolds have been widely studied and demonstrated great potential for enhancing bone tissue regeneration.3,4,15,16
Human bone marrow derived MSCs (hBM-MSCs) is currently the most reliable and frequently applied cell source for bone regeneration, however the low population of MSCs in bone marrow, particularly in older age patients, hampers their clinical application.10 Meanwhile, embryonic stem cells (ESCs) derived from the inner cell mass of a blastocyst have infinite proliferation capability and are pluripotent.17,18 However, concerns of immune rejection and ethical issues hinder their widespread usage.19
Induced pluripotent stem cells (iPSCs), generated by ectopically expression of four typical transcription factors (Oct4, Sox2, Klf4, and c-Myc)20,21 have emerged as an intriguing alternative for ESCs.12 As an important advance in stem cell research, which allows patient specific stem cell sources, iPSCs can further give rise to all kinds of progenitor cells, such as MSCs for bone and cartilage regeneration.10,20,21 Direct derivation of MSCs from pluripotent stem cells represents an effective alternative to obtain larger population of progenitor cells that are needed for cell therapies or regenerative medicine.22–26 Most importantly, iPSCs-derived MSCs have not only proved with efficient osteogenic function as MSCs harvested directly from adult tissue, but also demonstrated a higher proliferation capacity.27 Previously, we have developed a simple method for deriving functional human MSCs from iPSCs (hiPS-MSCs) generated by mRNA reprogramming,28 where full osteogenic functions of the hiPS-MSCs have been characterized.
Although hiPS-MSCs are proposed to have similar properties as MSCs, their osteogenic responses as an alternative for MSCs to nanofibrous scaffold has not been investigated yet. Therefore, in this study, the response of hiPS-MSCs to polycaprolactone (PCL) nanofibrous mats containing 44 wt% nHAp, with respect to neat PCL mats, was explored, using osteogenic genes (RUNX2, ALP, COL1A1, and OC) expression, alkaline phosphatase (ALP) activity, calcium deposition, as well as scanning electron microscopy (SEM). We hypothesize that (1) hiPS-MSCs are able to attach, proliferate, and differentiate on the nanofibrous scaffolds; and (2) supplement with bioactive nHAp in the scaffold will synergistically promote hiPS-MSCs osteogenic differentiation.
Materials and methods
Electrospinning
Pure polycaprolactone (PCL, MW = 80 kDa, Sigma) solution was prepared by dissolving PCL (20 w/v%) in dimethylformamide–chloroform (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) and stirring overnight at room temperature. In order to improve the miscibility and generate a homogenous solution prior to electrospinning, the solution of PCL with nano-hydroaxyapatite (nHAp) was prepared by dissolving PCL 10 w/v and nHAp (Sigma aldrich, particle diameter less than 200 nm) (8 w/v%) in hexafluoroisopropanol (HFIP) and stirring overnight at room temperature. The concentration of nHAp (44 wt%) was chosen on the previous literature,15 since further increase of nHAp content would render the fibers fragile and susceptible for breaking. The solutions were placed in a 3 mL syringe with a metallic needle (20G). The syringe was fixed horizontally on the syringe pump (model KDS101, KD Scientific), with 10 cm distance to the collector. An electrode of high-voltage power supply (Spellman High Voltage Electronics Corporation, MP Series) was clamped to the metal needle tip. The flow rate of polymer solution was 1.2 mL h−1, and the applied voltage was 7 kV for pure PCL, 5 kV for PCL–nHAp mixture, respectively. To generate electrospinning sheet with homogeneous thickness, the collecting shaft was attached to an AC motor, with rotation speed regulated by rheostat. The shaft was rotated at 500 rpm (1.4 m s−1 at the surface of the shaft). In total, 3 mL polymer solutions were electrospun and deposited on the collector as 50 mm × 150 mm rectangle sheets. Products were stored in vacuum desiccator before further use and characterizations.
3) and stirring overnight at room temperature. In order to improve the miscibility and generate a homogenous solution prior to electrospinning, the solution of PCL with nano-hydroaxyapatite (nHAp) was prepared by dissolving PCL 10 w/v and nHAp (Sigma aldrich, particle diameter less than 200 nm) (8 w/v%) in hexafluoroisopropanol (HFIP) and stirring overnight at room temperature. The concentration of nHAp (44 wt%) was chosen on the previous literature,15 since further increase of nHAp content would render the fibers fragile and susceptible for breaking. The solutions were placed in a 3 mL syringe with a metallic needle (20G). The syringe was fixed horizontally on the syringe pump (model KDS101, KD Scientific), with 10 cm distance to the collector. An electrode of high-voltage power supply (Spellman High Voltage Electronics Corporation, MP Series) was clamped to the metal needle tip. The flow rate of polymer solution was 1.2 mL h−1, and the applied voltage was 7 kV for pure PCL, 5 kV for PCL–nHAp mixture, respectively. To generate electrospinning sheet with homogeneous thickness, the collecting shaft was attached to an AC motor, with rotation speed regulated by rheostat. The shaft was rotated at 500 rpm (1.4 m s−1 at the surface of the shaft). In total, 3 mL polymer solutions were electrospun and deposited on the collector as 50 mm × 150 mm rectangle sheets. Products were stored in vacuum desiccator before further use and characterizations.
Generation of iPS-MSCs
Method for deriving functional hiPS-MSCs was fully described in our precious study.28 Briefly, primary human fibroblasts IMR90 (ATCC catalog CCL-186) were reprograming into iPSCs by mRNA reprogramming. RNAiMAX (Invitrogen) was used to deliver the modified mRNA reprogramming cocktail into fibroblasts. Clones of iPSCs were generated 18–20 days of modified mRNA transfection. Characterized iPSCs were further differentiated into hiPS-MSCs by our modified method. Three days after regulation passaging of iPSCs, iPSCs medium was replaced with MSCs derivation medium, which consisted of DMEM-low glucose (Biological Industries), 2 mM L-glutamine, 1% penicillin/streptomycin, and 10% FBS (Gibco). The MSC medium was changed every 2 days. After 14 days culture, the cells were trypsinized (0.25% trypsin/1 mM EDTA, Difco-Sigma) and expanded in MSCs medium on 0.1% gelatin-coated dishes (Becton Dickinson). Homogeneous population of fibroblast-like MSCs was obtained after three passagings.Cell proliferation rate assay
Cell proliferation rate was conducted with flow cytometry using the Click-iT EdU flow cytometry assay kit (Cat. no. C10425, Lifetechnologies). hiPS-MSCs were from our previous study.28 The hiPS-MSCs (passage 7) and hBM-MSCs (Lonza PT 2501, passage 7) were seeded in 6-well plates (1.0 × 105 cells per well) 24 hours prior changing to fresh growth medium containing 10 μM EdU. Cells cultured in growth medium without EdU were used as negative controls. Cells were incubated at 37 °C in a humidified incubator with 5% CO2 for 2 hours, then harvested by trypsinization and proceeded to EdU staining according to the manufacturer's protocol. Briefly, cells were in order washed with 500 μL 1% BSA-PBS, incubated with 100 μL Click-iT fixative for 15 min in dark, washed with 500 μL 1% BSA-PBS, re-suspended with 100 μL 1× component E, stained in labeling cocktail for 30 min, washed with 500 μL 1× component E, and analyzed with flow cytometry (BD FACSCalibur).Cell growth curve
The hiPS-MSCs (passage 7) and hBM-MSCs (passage 7) were seeded in a 12-well plate at 3000 and 6000 cells per cm2, and cultured in growth mediums at 37.5 °C, 5% CO2. After 2, 4, 6 days, the cells were harvested by trypsinization and counted by Hemocytometer.Scaffolds seeding and proliferation assay
The scaffolds were punched out from the electrospun sheet by a biopsy punch with 5 mm diameter (Miltex). Scaffolds were disinfected and rehydrated with decreasing concentrations of ethanol (100%, 70%, 50%, 30%; 30 min per step), then washed with PBS three times and incubated in growth medium overnight. 96-well culture plates were coated with 1% agarose before placing the scaffolds. hiPS-MSCs, passage 7 were seeded in 20 μL cell suspension containing 2 × 104 cells, incubated at 37.5 °C, 5% CO2 for 2 hours before adding 200 μL per scaffold growth medium. The same amount cells seeded on the culture plate were as control. Media were changed every 3 days for a culture period of 24 days. Cell proliferation was measured at day 3, 8, 16, and 24, using the XTT assay from Roche (Cat #: 11465015001, Roche) according to the manufacturer's instructions. Briefly, 150 μL XTT solution was added to the cells and incubate for 4 hours at 37.5 °C, 5% CO2. The orange formazan dye from yellow tetrazolium salt XTT by metabolic active cells was measured at 450 nm using a Victor3 1420 Multilabel Counter (PerkinElmer, Waltham, MA). Cells were gently washed with growth medium, and 200 μL growth medium was added for subsequent culturing. To investigate the cell adherence and spread, cell loaded structures were mounted in VectaShield with DAPI (Vector Laboratories). Images were captured with a fluorescence microscope using image software (LEICA DMIEBE).Osteogenic differentiation of hiPS-MSCs on scaffolds
The scaffold preparation and cell seeding procedures were the same as above. After 3 days, the growth medium was changed to either growth medium or differentiation medium containing DMEM-high glucose (Invitrogen), 10% FBS (Gibco), 1% penicillin/streptomycin, 100 nM dexamethasone (D2915, Sigma), 10 mM β-glycerophosphate (G9891, Sigma), and 50 μM ascorbic acid-2-phosphate (A8960, Sigma), and 10 μM Vit-D (D1530, Sigma). Then all media were changed every 3 days for a culture period of three weeks.qPCR
Total RNA was extracted from cell/scaffold constructs with an RNeasy Mini Kit (Qiagen) according to the manufacturer's instructions, followed by quantification with a Nanodrop spectrophotometer and qualification by 1% agarose gel electrophoresis. For first-strand DNA synthesis, approximately 15–85 ng of total RNA were reverse transcribed using a cDNA synthesis kit (170-8891, Bio-Rad, Hercules, CA) in a final volume of 20 μL. Q-PCR primers for RUNX2, ALP, COL1A1, OC and GAPDH are listed in Table 1 (ESI data†). Two μL (five times diluted) cDNA was used for RT-qPCR analysis. RT-qPCR was performed using the LightCycle 480 SYBR Green I Master kit on LightCycler 480 (Roche) with the following PCR program: 1 hold at 95 °C for 5 min; 50 cycles at 95 °C for 10 s, 60 °C for 10 s, and 72 °C for 15 s. To mask the signal from primer dimers, fluorescent signal was acquisited once at 77 °C for RUNX2, 80 °C for ALP, 82 °C for GAPDH and COL1A1, and 84 °C for OC. Relative gene expression was calculated using the 2−ΔΔct method after normalization to the reference gene GAPDH.ALP activity and calcium assay on cell-seeded scaffold
hiPS-MSCs-seeded scaffolds were transferred to 200 μL DMEM w/o Phenol Red (Gibco 21063) and sonicated at intervals of 1 s on and 5 s off with amplitude of 50% (0.046 kJ) in a Bandelin Sonopuls sonicator (Buch & Holm, Denmark) for a total of 1 min. An aliquot of 60 μL was used for ALP activity assay. The remaining solution was mixed with an equal volume of 1 M acetic acid and incubated on a shaker overnight to dissolve the calcium deposition. ALP activity was measured using a colorimetric endpoint assay measuring the enzymatic conversion of p-nitrophenyl phosphate (Sigma-Aldrich) to the yellowish product p-nitrophenol in the presence of ALP. Absorbance of p-nitrophenol was measured in a microplate reader (Victor3 1420 Multilabel Counter, PerkinElmer Life Sciences, Denmark) at 405 nm. Calcium contents were quantified by a colorimetric endpoint assay based on the complex formation of one Ca2+ ion with two Arsenazo III molecules to a blue-purple product (Diagnostic Chemicals Limited, Charlottetown, PE, Canada). Ca2+ concentration was quantified spectrophotometrically at 650 nm. Considering the possibility of some fragments of the scaffolds with the nHAp component might be present and affect the Ca2+ measurement, we used the de novo calcium content by subtracting the calcium content from blank scaffolds in proliferation cultures.SEM
The samples were examined using environmental mode SEM (Nova NanoSEM 600; FEI Company, Hillsboro, OR) and the mineral secreted by the cells was analyzed by means of an energy dispersive X-ray spectrometer (EDX). To determine fiber diameters, SEM images were analyzed using the image J software. The mean fiber diameter was determined by measuring the diameters of 100 randomly selected fibers. Three cell loaded scaffolds from each group at week 3 were prepared by fixing in 2.5% glutaraldehyde containing 0.1 M sodium cacodylate buffer, dehydrating in a graded ethanol series and air-dried. Images were taken at randomly selected 3 parts of each sample.Statistics
Data from XTT test, qPCR, ALP activity, and calcium assay are presented as mean ± standard deviation (SD). Statistical differences were determined using ANOVA variance and Student's t-test. P-values less than 0.05 were considered significant.Results and discussion
MSCs showed superior interactions with nanoscale substrate and osteogenic response with induction signals such as the nHAp.29–32 As a novel source of MSCs, the hiPS-MSCs, their interactions, including cell proliferation, osteogenic genes expression, ALP activity and calcium deposition, with the nHAp contained nanofibrous scaffolds were studied in this work.The composite nanofibers were successfully prepared by electrospinning method. The morphological and chemical characterization were analyzed by SEM/EDX (Fig. 1). The PCL fibers have an average diameter of 2.21 ± 0.39 μm (Fig. 1a); the PCL–nHAp fibers have an average diameter 0.96 ± 0.48 μm, where nHAp particles were homogeneous dispersed in the fibers without causing any rupture (Fig. 1b). EDX analysis confirmed the presence of Ca and P, originated from the bioactive component nHAp (Fig. 1c and d).
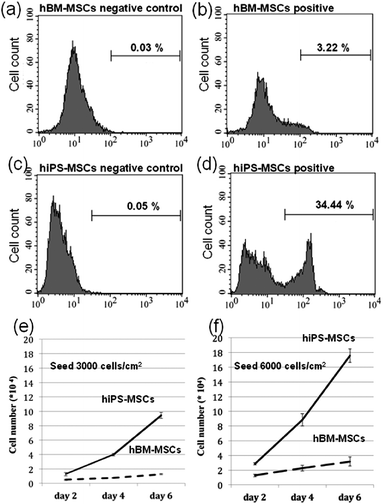
The hiPS-MSCs used for this study were generated with protocol described by Yen et al.17 and we have characterized MSCs markers by flow cytometry and their tri-lineages differentiations capacity in our previous study. In this study, the advantage of hiPS-MSCs self renewal capability was further confirmed by the cell proliferation rate of 34.44% with 10 times higher than 3.22% of hBM-MSCs. Moreover, the cell number increasement during 6 days culture also confirmed that hiPS-MSCs proliferate faster than hBM-MSCs (Fig. 2).
Investigation of the cell viability for evaluation of the biocompatibility of biomaterials is very important. Significant increase of XTT value in both scaffolds during the three weeks culture period indicated the biocompatibility of the bioactive scaffods for hiPS-MSCs proliferation (Fig. 3c). Moreover, the cells were found adherent and well spreaded on both fibers at week 3, visualized by DAPI staining (Fig. 3a and b). XTT data shows that the cells are confluent at week 3; since the surface of the electrospun sheet is not flat, there is often some area out of the focus under the fluorescence microscope, and the DAPI images don't demonstrate the true confluency of the cells. On the other hand, ECM formation on the fibers can be clearly viewed by SEM imaging (Fig. 4a–f). The final ECM formation containing Ca and P elements analysed by EDX suggests the suitability of the scaffolds to promote ECM calcification (Fig. 4g and h). These were consistent with previous studies where favorable cell–scaffold interactions on electrospun scaffolds for stem cells, fibroblasts were found.15
Furthermore, the osteogenic gene expression was assessed (Fig. 5). Significant higher expression of RUNX2, ALP, COL1A1, and OC were observed in both scaffolds in differentiation medium than in growth medium. Even though the highest gene expression levels of RUNX2, ALP and COL1A1 were observed at different time points between the two scaffolds, the OC gene expression in both scaffolds had the same profiles with highest expression at week 3. Significant higher increase of OC gene expression was observed in PCL–nHAp scaffold compared with PCL scaffold in differentiation medium (P = 0.0207 at week 3), even in the growth medium (P = 0.032 at week 2). Therefore, the nHAP contained scaffold itself has demonstrated an osteogenic induction on the hiPS-MSCs.
To further analyses differentiation of hiPS-MSCs, ALP activity and calcium deposition were assessed. ALP is an early marker and expressed by cells during the early stages of osteogenic differentiation. A substantial increase in ALP activity level was observed in both scaffolds cultured in differentiation medium compared with growth medium, irrespective of scaffold types (PCL P < 0.0001; PCL–nHAp P = 0.0039), suggesting osteoblastic differentiation of hiPS-MSCs in osteogenic medium toward the osteogenic lineage. Interestingly, significantly increased ALP activity was again observed in the PCL–nHAp scaffold in growth medium (P = 0.0154). The osteogenic induction of the bioactive nanofibrous scaffolds was found consisted with pervious studies.16,33
The osteogenic induction of the bioactive nHAp on the hiPS-MSCs differentiation was also evidenced by the significantly increased de novo calcium deposition, (199.57 ± 111.10 μg in PCL and 968.34 ± 311.01 μg in PCL–nHAp, P = 0.0035. Fig. 6) which was recognized as the main functional component in bone matrix secreted in the later period of osteogenic differentiation. This also confirmed with higher OC gene expression in PCL–nHAp scaffold.
Therefore, the biomimic PCL–nHAp nanofibrous scaffolds promote hiPS-MSCs to produce more calcified structure for bone tissue regeneration. For clinical applications, further improved scaffold designs for bone tissue engineering, as well as the safety of hiPS-MSCs with both in vitro and in vivo models are still ongoing.
Conclusions
In this study, the interaction between biomimic PCL–nHAp nanofibrous scaffolds and the hiPS-MSCs were investigated. In general, our results suggested that the hiPS-MSCs are able to adhere, proliferate and osteogenic differentiate to form calcified structure on electrospun nanofibrous scaffolds, while the addition of nHAp further enhances osteogenic differentiation potential of hiPS-MSCs.Acknowledgements
The authors would like to thank Anette Baatrup for her great technical assistance. This project is supported by grants from Danish Strategic Research Foundation on Project ElectroMed (11-115313), Velux (25906), Lundberk Foundation, International cooperation and Natural Science Foundation of Jiansu province of PRC (BZ2011046, BK2012490).Notes and references
- A. L. Carlson, C. A. Florek, J. J. Kim, T. Neubauer, J. C. Moore, R. I. Cohen, J. Kohn, M. Grumet and P. V. Moghe, FASEB J., 2012, 26, 3240–3251 CrossRef CAS PubMed.
- A. Shafiee, M. Soleimani, G. A. Chamheidari, E. Seyedjafari, M. Dodel, A. Atashi and Y. Gheisari, J. Biomed. Mater. Res., Part A, 2011, 99, 467–478 CrossRef PubMed.
- J. H. Jang, O. Castano and H. W. Kim, Adv. Drug Delivery Rev., 2009, 61, 1065–1083 CrossRef CAS PubMed.
- S. Sahoo, L. T. Ang, J. C. Goh and S. L. Toh, J. Biomed. Mater. Res., Part A, 2010, 93, 1539–1550 Search PubMed.
- W. Ji, Y. Sun, F. Yang, J. J. van den Beucken, M. Fan, Z. Chen and J. A. Jansen, Pharm. Res., 2011, 28, 1259–1272 CrossRef CAS PubMed.
- A. Abdal-hay, F. A. Sheikh and J. K. Lim, Colloids Surf., B, 2013, 102, 635–643 CrossRef CAS PubMed.
- G. Sui, X. Yang, F. Mei, X. Hu, G. Chen, X. Deng and S. Ryu, J. Biomed. Mater. Res., Part A, 2007, 82, 445–454 CrossRef PubMed.
- K. Fox, P. A. Tran and N. Tran, ChemPhysChem, 2012, 13, 2495–2506 CrossRef CAS PubMed.
- S. M. Zakaria, S. H. Sharif Zein, M. R. Othman, F. Yang and J. P. D. Jansen, Tissue Eng., Part B, 2013, 19, 431–441 CrossRef CAS PubMed.
- S. Diederichs, K. M. Shine and R. S. Tuan, BioEssays, 2013, 35, 220–230 CrossRef CAS PubMed.
- M. F. Pittenger, A. M. Mackay, S. C. Beck, R. K. Jaiswal, R. Douglas, J. D. Mosca, M. A. Moorman, D. W. Simonetti, S. Craig and D. R. Marshak, Science, 1999, 284, 143–147 CrossRef CAS.
- S. M. Wu and K. Hochedlinger, Nat. Cell Biol., 2011, 13, 497–505 CrossRef CAS PubMed.
- K. A. Keyser, K. E. Beagles and H. P. Kiem, Cell Transplant., 2007, 16, 555–562 Search PubMed.
- K. J. Beggs, A. Lyubimov, J. N. Borneman, A. Bartholomew, A. Moseley, R. Dodds, M. P. Archambault, A. K. Smith and K. R. McIntosh, Cell Transplant., 2006, 15, 711–721 Search PubMed.
- E. H. van Manen, W. Zhang, X. F. Walboomers, B. Vazquez, F. Yang, W. Ji, N. Yu, D. J. Spear, J. A. Jansen and P. C. Yelick, Odontology, 2012, 93, 1539–1550 Search PubMed.
- H. Peng, Z. Yin, H. Liu, X. Chen, B. Feng, H. Yuan, B. Su, H. Ouyang and Y. Zhang, Nanotechnology, 2012, 23, 485102 CrossRef CAS PubMed.
- M. L. Yen, C. H. Hou, K. Y. Peng, P. C. Tseng, S. S. Jiang, C. T. Shun, Y. C. Chen and M. L. Kuo, Cell Transplant., 2011, 20, 1529–1545 Search PubMed.
- T. Ozdemir, A. M. Higgins and J. L. Brown, Curr. Pharm. Des., 2013, 19, 3446–3455 CrossRef CAS.
- E. Kiskinis and K. Eggan, J. Clin. Invest., 2010, 120, 51–59 CrossRef CAS PubMed.
- K. Takahashi, K. Tanabe, M. Ohnuki, M. Narita, T. Ichisaka, K. Tomoda and S. Yamanaka, Cell, 2007, 131, 861–872 CrossRef CAS PubMed.
- K. Takahashi and S. Yamanaka, Cell, 2006, 126, 663–676 CrossRef CAS PubMed.
- L. G. Villa-Diaz, S. E. Brown, Y. Liu, A. M. Ross, J. Lahann, J. M. Parent and P. H. Krebsbach, Stem Cells, 2012, 30, 1174–1181 CrossRef CAS PubMed.
- Y. Liu, A. J. Goldberg, J. E. Dennis, G. A. Gronowicz and L. T. Kuhn, PLoS One, 2012, 7, e33225 CAS.
- M. Giuliani, N. Oudrhiri, Z. M. Noman, A. Vernochet, S. Chouaib, B. Azzarone, A. Durrbach and A. Bennaceur-Griscelli, Blood, 2011, 118, 3254–3262 CrossRef CAS PubMed.
- G. Bilousova, H. Jun du, K. B. King, S. De Langhe, W. S. Chick, E. C. Torchia, K. S. Chow, D. J. Klemm, D. R. Roop and S. M. Majka, Stem Cells, 2011, 29, 206–216 CrossRef CAS PubMed.
- T. Teramura, Y. Onodera, T. Mihara, Y. Hosoi, C. Hamanishi and K. Fukuda, Cell. Reprogramming, 2010, 12, 249–261 CrossRef CAS PubMed.
- Q. Lian, Y. Zhang, J. Zhang, H. K. Zhang, X. Wu, F. F. Lam, S. Kang, J. C. Xia, W. H. Lai, K. W. Au, Y. Y. Chow, C. W. Siu, C. N. Lee and H. F. Tse, Circulation, 2010, 121, 1113–1123 CrossRef PubMed.
- L. Zou, Y. Luo, M. Chen, G. Wang, M. Ding, C. C. Petersen, R. Kang, F. Dagnaes-Hansen, Y. Zeng, N. Lv, Q. Ma, D. Q. Le, F. Besenbacher, L. Bolund, T. G. Jensen, J. Kjems, W. T. Pu and C. Bunger, Sci. Rep., 2013, 3, 2243 Search PubMed.
- S. A. Kuznetsov, N. Cherman and P. G. Robey, Stem Cells Dev., 2011, 20, 269–287 CrossRef CAS PubMed.
- B. Leukers, H. Gulkan, S. H. Irsen, S. Milz, C. Tille, M. Schieker and H. Seitz, J. Mater. Sci.: Mater. Med., 2005, 16, 1121–1124 CrossRef CAS PubMed.
- R. Shu, R. McMullen, M. J. Baumann and L. R. McCabe, J. Biomed. Mater. Res., Part A, 2003, 67, 1196–1204 CAS.
- X. Guo, J. E. Gough, P. Xiao, J. Liu and Z. Shen, J. Biomed. Mater. Res., Part A, 2007, 82, 1022–1032 CrossRef PubMed.
- V. D'Anto, M. G. Raucci, V. Guarino, S. Martina, R. Valletta and L. Ambrosio, J. Tissue Eng. Regener. Med., 2013 DOI:10.1002/term.1768.
Footnote |
| † Electronic supplementary information (ESI) available: Detail primer sequences for PCR analysis. See DOI: 10.1039/c3ra44181d |
| This journal is © The Royal Society of Chemistry 2014 |