Oxidative dehydrogenation of ethylbenzene to styrene over zirconium vanadate catalyst prepared by solution combustion method
Akrati
Verma
,
Reena
Dwivedi
,
Prabhakar
Sharma
and
R.
Prasad
*
Catalysis & High Pressure Lab, School of Chemical Sciences, Devi Ahilya University, Indore, India. E-mail: rjndr.prsd3@gmail.com; Fax: +91 731-2365782; Tel: +91 9575010982
First published on 14th October 2013
Abstract
A new type of mesoporous nanocrystalline vanadium incorporated zirconia material is synthesized by solution combustion method. The material is characterized by X-ray diffractometer (XRD), Brunanuer, Emmett and Teller (BET) surface area, Raman spectroscopy, and transmission electron microscopy (TEM) techniques. The XRD studies revealed the material to be a mixture of ZrO2 and ZrV2O7. The effect of calcination temperature on the phase transformation of the materials has been investigated by XRD measurements. The material has been used as catalyst for oxidative dehydrogenation (ODH) of ethylbenzene (EB) using air as oxidant for the first time. A DFT modeling of the transition state of the reaction suggests the formation of a hydroxyl species over the catalyst surface which is believed to have been formed due to abstraction of a hydrogen atom from ethyl benzene. The presence of hydroxyl species on the catalyst surface has also been identified by FTIR record of the catalyst after treating it with ethyl benzene at 200 °C. A tentative mechanism for the reaction is proposed.
1. Introduction
Oxidative dehydrogenation (ODH) of ethyl benzene (EB) to styrene (S) is a commercially important process because styrene and its derivatives find applications as a raw material for many commercial products such as styrene oxide, polystyrene, polystyrene oxide and polycarbonates. Styrene oxide can also be reduced to phen-ethyl alcohol, a bulk perfume used in many perfume formulations. Conventional method of producing styrene involves dehydrogenation of EB over Fe–K–Cr catalysts, which is energy intensive and requires super heated steam.1 Besides, the process leads to formation of coke over the catalyst surface, and conversion is also less than 50% per pass. Efforts are therefore made to find an alternative process and catalyst for production of styrene. Vanadium catalysts based ODH seems to be a better alternative for the conventional dehydrogenation because it is exothermic and requires a lower temperature. The energy released during reaction can be used for other applications such as steam generation and production of electricity.Pereira et al. achieved around 69% styrene relative yield with an activated carbon catalyst using air as an oxidant at 350 °C.2 Jie Xu et al. synthesized mesostructured CeO2 and used it as an effective catalyst for styrene synthesis and achieved 85–86% styrene yield, using O2/N2 as an oxidant in the temperature range of 350–550 °C.3 Celis et al. derived activated carbon from a native wood and performed the ODH of ethylbenzene to styrene and achieved 40–45% styrene yield at 350 °C.4 Shiju et al. also performed the same reaction using VOx/Al2O3 catalyst with N2O as an oxidant at 500 °C and found 45% styrene yield.5 Gopinath et al. has explored vanadium incorporated titania catalyst and achieved 85% of styrene yield using oxygen as an oxidant at 500 °C.6 Chang et al. reported around 70% of styrene yield over supported vanadium antimony oxide catalyst with carbon dioxide as an oxidant at 550 °C.7
Different metal oxides such as Al2O3, TiO2, SiO2, ZrO2, MgO and HfO2 (ref. 8–12) can be used as support for V2O5. ZrO2 supported V2O5 (VOx/ZrO2) catalyst is a better choice because of its chemical and thermal stability, strong support–catalyst interaction and good dispersion.13 Method of preparation,14 vanadium loading,15 interaction and nature of the support16,17 are detrimental factors in the catalytic performance of the VOx/ZrO2 catalyst.18 It is a potential catalyst for many industrial reactions such as selective oxidation of hydrocarbons,19–24 oxidative dehydrogenation of propane and butane,25–27 oxidation of methanol28 and toluene,29 reduction of NO with NH3,30,31 ammoxidation of alkylaromatics.32,33
The solution combustion synthesis (SCS) is one of the important methods for the preparation of mesoporous materials.34 In this method a solution of nitrate salts of metals is heated along with an organic fuel such as urea, citric acid which results in self-firing, generation of intense heat and instantaneous rise in temperature.35 SCS is a simple, low-cost and rapid process, permitting synthesis of a variety of nanosized materials.
To the best of our knowledge, there is no report on the synthesis of zirconium vanadate employing solution combustion method and its application as a catalyst for ODH of ethylbenzene. The present problem was therefore undertaken with a view (1) to develop a cheap and fast solution combustion assisted synthesis of zirconium vanadate, (2) to characterize it by employing various physicochemical methods such as XRD, BET surface area measurement, Raman, SEM and TEM, (3) to test its performance as catalyst for oxidative dehydrogenation of ethylbenzene, (4) to optimize the process conditions for maximum yield of styrene, and (5) to propose a tentative mechanism.
2. Experimental details
2.1. Catalyst preparation
Meso-structured vanadium doped zirconium materials were synthesized via a previous established solution combustion method.6 High purity, zirconium oxychloride (S.D. Fine Chemicals), ammonium metavanadate and urea (LOBA Chemie Pvt. Ltd.) were used for the preparation of the zirconium vanadate (ZrV2O7) nanoparticles. In a typical preparation a solution of zirconium nitrate, (prepared by mixing 5.8 g of zirconium oxychloride with 12 ml of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 HNO3) is mixed with another solution prepared by mixing 0.23 g of NH4VO4 in 50 ml of water. The final solution was mixed with 1.2 g of urea and fired in a microwave oven for five minutes. The material swells into a yellow colored gel. The product obtained was grinded and kept for calcinations in a muffle furnace at a temperature of 500 °C for 4 h. On calcinations, a green colored residue was obtained which was grinded in a motor-pastel to make a fine powder. All preparations are coded as ZVX where X represents the percentage (%) of vanadium oxide in the sample.
2 HNO3) is mixed with another solution prepared by mixing 0.23 g of NH4VO4 in 50 ml of water. The final solution was mixed with 1.2 g of urea and fired in a microwave oven for five minutes. The material swells into a yellow colored gel. The product obtained was grinded and kept for calcinations in a muffle furnace at a temperature of 500 °C for 4 h. On calcinations, a green colored residue was obtained which was grinded in a motor-pastel to make a fine powder. All preparations are coded as ZVX where X represents the percentage (%) of vanadium oxide in the sample.
2.2. Catalyst characterization
XRD measurements of zirconium vanadate was made using Rigaku X-ray powder diffractometer using Cu-Kα radiation with wavelength 1.5406 Å as a source The diffractometer was equipped with a graphite crystal monochromator (for the diffracted beam) and scintillation counter as detector. Surface area was measured using ASAP 2020 V3.04 H, surface area analyzer in a N2 physisorption apparatus equipped with a thermal conductivity detector. The pore size distribution was calculated using BJH method. Raman records in the range 50–4000 cm−1 were made on a Labram HR800 micro Raman spectrometer using 488 nm wavelength Ar+ laser source at the energy of 2.53 eV and using Labspec software. TEM records were made using Technai G2 20 microscope.2.3. Catalytic activity
The activity of the catalysts for oxidative dehydrogenation of styrene was measured in the temperature range of 350–550 °C at atmospheric pressure using a vertical down flow reactor, placed in a tubular furnace. Temperature of the furnace was measured with the help of a thermocouple. Details of reactor set-up are described elsewhere.36,37 1.0 gm of catalyst was packed in between two layers of inert glass beads. The reactant (EB) was fed using a syringe at WHSV of 2 ml h−1. Air was used as an oxidant, and its optimum flow rate was 60 ml min−1.Prior to reaction, the catalyst was activated in a flow of air at 500 °C for 30 minutes. Reaction products were collected and analyzed by a Gas Liquid Chromatograph (Chemito Gas Chromatograph 7610) equipped with Flame Ionization Detector.
3. Results and discussion
3.1. X-Ray diffraction analysis
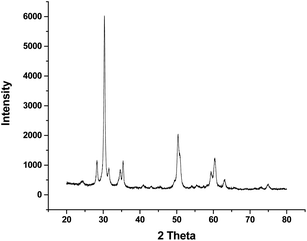
Powder XRD patterns of the prepared nanoparticles calcined at different temperatures were recorded in order to explore structural features of zirconium supported mesoporous vanadium materials. Fig. 1 depicts XRD patterns of as prepared ZrO2 and ZVX materials in the 2θ range of 20 to 80 degrees, mainly tetragonal (2θ = 30.2 degree) and monoclinic (2θ = 31.6 degree) phases of zirconia were detected. The pattern indexed matches with the tetragonal ZrO2 (JCPDS card file no. 81-1551) and cubic ZrV2O7 (JCPDS card file no. 16-0422). The absence of any vanadia peaks (2θ = 20.3 degree) in the sample calcined at 400 °C and 600 °C and the appearance of the vanadia peaks with lower intensity in the sample calcined at 800 °C confirms that the vanadium ions have occupied the zirconium ions at their lattice positions and high dispersion of vanadia ions on zirconium oxide surface.38 On calcinations at higher temperature the full width at half maximum of the reflection peaks decreases, suggests that the crystal size of prepared zirconium vanadate nanoparticles is increasing with rising temperature. When zirconium vanadate sample were calcined at 400 °C and 600 °C a very sharp peak appeared at 30.23 degrees which can be ascribed to tetragonal phase of ZrO2 and on calcinations at 800 °C peaks appeared at 28.2 degrees and 31 degrees in the sample calcined at 800 °C can be ascribed to monoclinic phase of ZrO2.39−41 The average particle size was calculated from (111) diffraction peak using Scherer's equation D = 0.9λ/(β![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cos
cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ), where D is the average crystallite size in nm, λ is the wavelength of source X-ray (0.154 nm), β (in radian) is the full peak width at half maximum. The average particle size found in the range of 20 to 26 nm.
θ), where D is the average crystallite size in nm, λ is the wavelength of source X-ray (0.154 nm), β (in radian) is the full peak width at half maximum. The average particle size found in the range of 20 to 26 nm.
 | ||
| Fig. 1 Representative powder XRD pattern for ZrO2 nanoparticles calcined at 450 °C and ZV10 catalyst calcined at (A) 400 °C, (B) 600 °C, (C) 800 °C. | ||
| Sample | Surface area (m2 g−1) | Pore size (nm) | Pore volume (ml g−1) |
|---|---|---|---|
| ZV2 | 2.93 | 3.13 | 0.002 |
| ZV5 | 10.55 | 7.7 | 0.013 |
| ZV8 | 25.82 | 4.5 | 0.022 |
| ZV10 | 35.65 | 3.5 | 0.027 |
3.2. BET surface area
The nitrogen BET surface areas of various samples calcined at 400 °C are summarized in Table 1. The above data were collected to investigate the textural properties of the ZVX materials. N2 adsorption–desorption isotherms of ZVX samples are shown in Fig. 2. The isotherms were all of type IV as per IUPAC classification and all the materials show H2 hysteresis loop which is characteristic for all the mesoporous material.42 As the vanadium concentration increases the hysteresis loop became more pronounced. The pore size distribution was found in the range of 3.13 to 7.70 nm. Catalyst containing 10% vanadium possessed maximum surface area and was found to perform best. | ||
| Fig. 2 N2 adsorption–desorption isotherms of ZVX samples of (A) ZV2 (B) ZV5, (C) ZV8 and (D) ZV10 calcined at 400 °C. | ||
| Observed | Ref. 39 | Ref. 44 | Ref. 46 | Assignments |
|---|---|---|---|---|
| a ν = stretching, s = symmetry, w = week, m = medium, s = strong, mc = monoclinic, β = bending. | ||||
| 144.8(s) | 156 | 144 | Lattice | |
| 330(w) | 331(m) | Tetragonal ZrO2 | ||
| 407(m) | 404 | β (VO4) | ||
| 482(s) | 490(mc) | 477(m) | ZrO2 | |
| 639(w) | 640(mc) | 636(m) | ZrO2 | |
| 784(w) | 780(s) | 774 | ν s (tetrahedron VO4) | |
| 888(s) | 860 | 874 | ν sV–O–V (tetrahedron VO4) | |
3.3. Raman
The Raman spectra of ZrO2 and ZV10 sample calcined at 400° C are reproduced in Fig. 3(A) and (B) respectively. The assignments of observed Raman bands are made on the basis of reported assignments39,41–46 and are shown in Table 2. The bands appeared at 330, 482 and 639 cm−1 are assigned to ZrO2. The low frequency bands appeared at 144.8, 175.9 and 188 cm−1 are assigned to lattice vibrations. Bands appeared at 1372 and 1597 cm−1 can be attributed to bending modes of water. The band appeared at 1042 cm−1 in the Raman spectrum of ZV10 can be ascribed to stretching vibration of short V![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond. A strong Raman band at 996 cm−1 is generally assigned to V
O bond. A strong Raman band at 996 cm−1 is generally assigned to V![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching mode of bulk V2O5.47,48 Weak intensity of this band in the present recording suggests low concentration of bulk V2O5.
O stretching mode of bulk V2O5.47,48 Weak intensity of this band in the present recording suggests low concentration of bulk V2O5.
3.4. Transmission electron microscopy (TEM)
Transmission electronic microscopy (TEM) in high-resolution mode is the best tool to analyze the morphology and sizes of the prepared nanoparticles.49,50 TEM micrographs taken for the ZV10 samples calcined at 400 °C are shown in Fig. 4. The TEM particle size was found in the range of 10–40 nm. The corresponding electron diffraction pattern is shown in Fig. 4(B). The image shows well crystallized ZV10 nanoparticles with no indication of any clear amorphous phase. Therefore, it is inferred that vanadia is in a highly dispersed state in the ZrO2 lattice in ZV10 materials.27 The presence of the reflections in the electron diffraction (ED) pattern and broadening of the rings can be ascribed to the presence of small randomly oriented V–Zr mixed oxide particles. Extra spots appearing in the pattern can be ascribed to the formation of new phases during heat treatment.514. Catalytic activity
4.1. Effect of reaction temperature
Effect of reaction temperature on conversion of EB and yield and selectivity of styrene over ZV10 catalyst is shown in Fig. 5. The conversion, selectivity and yield increases rapidly with the reaction temperature up to 550 °C and then decreases. The spent catalyst was black in colour. The decrease performance of the catalyst at temperature higher than 550 °C can be attributed to coke deposition at the catalyst surface6 and sintering of the catalyst.3 The effect of temperature on the selectivity of the styrene and by products is shown in Table 3. | ||
| Fig. 5 Effect of temperature on conversion of EB, selectivity and yield of styrene. Catalyst ZV10, EB = 2 ml h−1, air = 60 ml min−1. | ||
| S. no. | Temp. (°C) | Styrene | Benzene | Toluene | Styrene oxide |
|---|---|---|---|---|---|
| 1 | 400 | 67.60 | 1.67 | 12.6 | 1.4 |
| 2 | 450 | 72.16 | 2.62 | 4.32 | 1.23 |
| 3 | 500 | 74.62 | 0.09 | 0.08 | 0.86 |
| 4 | 550 | 79.90 | 2.14 | 0.86 | 0.78 |
4.2. Effect of vanadium concentration in the catalyst
Effect of percentage of vanadium (V) in the catalyst on the performance of ZVX is shown in Fig. 6. The catalytic activity of the present mesostructured ZVX catalysts were explored in the temperature range 400–550 °C, at EB flow rate of 2 ml h−1 and air flow of 60 ml h−1. EB conversion and yield of styrene increased with increase in V incorporation and reached a maximum with ZV10 catalyst and further increase in the vanadium concentration leads to decrease in EB conversion along with selectivity and yield of styrene. The most likely reason behind this result is the increase in near neighboring V–V interactions which may further lead to increase in total oxidation. | ||
| Fig. 6 Effect of wt% of vanadium on the performance of ZVX catalyst in the ODH of EB. Temp. = 550 °C, EB = 2 ml h−1, air flow = 60 ml min−1. | ||
4.3. Effect of EB flow and oxygen flow
EB conversions, selectivity and yield of styrene for ZV10 catalysts, at reaction temperature of 550 °C, with air as an oxidant has been examined thoroughly. Caution was taken during the reaction as air was used as an oxidant and is combustible nature of the reactant and the product along with the exothermic nature of the reactions. EB flow was kept constant at 2 ml h−1 for all the air flow variations and air flow rate was kept constant at 60 ml min−1 for all the EB flow variations. Fig. 7 depicts the effects of EB flow variations at fix air flow. The decrease in conversion with increasing flow rate of EB could be due to reduced time of contact between reactant and the active sites which ultimately results in a decrease in yield too. The optimum flow rate of EB was found to be 2 ml h−1. Flow rate of air was also one of the major factors for the ODH of EB to styrene. Air flow variation with constant EB flow rate (2 ml h−1) is shown in Fig. 8. Here it is found that EB conversion and styrene yield increases with the increase in air flow from 10 to 60 ml min−1. Further increase in air flow rate leads to a decrease in selectivity and yield but an increase in conversion. The optimum air flow rate was found to be 60 ml min−1. The decrease in selectivity at air flow rate >60 ml min−1 can be attributed to partial oxidation of styrene and reduction in coke formation.6 | ||
| Fig. 7 Effect of EB flow rate on performance of ZV10 catalyst. Air flow = 60 ml min−1, Temp. = 550 °C. | ||
5. Study of the spent catalyst and its correlation to activity
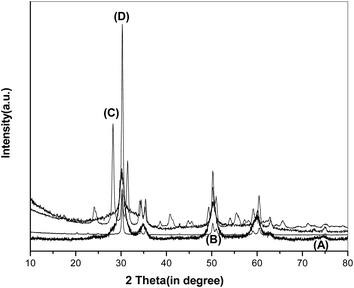
XRD and Raman study of the ZV10 spent catalyst after 5 hours of use at 550 °C in the ODH of EB with the air as oxidant is made in order to explain its structure activity relationship. XRD pattern of the spent catalyst is reproduced in Fig. 9. The pattern is similar to that of the fresh catalyst except that few extra peaks appeared at 2θ values of 24.4 degree and 28.3 degree. These peaks are assigned to the nanocrystalline carbon nanotubes.52 Deposition of carbon on the surface of the catalyst leads to a decrease in the activity. The absence of any vanadia peak even after this exothermic reaction, confirms the incorporation of active V2O5 species into zirconia lattice. Small shifting of the V![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching band of V2O5 in the Raman spectrum of the spent catalyst in Fig. 10 emphasizes no segregation of active phase due to the reaction. From this it can be concluded that active vanadia is well incorporated into the ZrO2 supports which prevents any agglomeration and segregation due to the exothermic reaction.
O stretching band of V2O5 in the Raman spectrum of the spent catalyst in Fig. 10 emphasizes no segregation of active phase due to the reaction. From this it can be concluded that active vanadia is well incorporated into the ZrO2 supports which prevents any agglomeration and segregation due to the exothermic reaction.
6. Mechanism of ODH reaction
Most of the mechanisms suggested for oxidative dehydrogenation of organic compounds over supported vanadium catalysts are based on Mars–van Krevelen redox mechanism53 and the C–H bond activation step is the rate determining step.54 In this mechanism the ZrO2/V2O5 catalyst is first reduced by the hydrocarbon which is subsequently re-oxidized by the oxidant. In order to confirm this kind of mechanism being operative in the present case we passed ethylbenzene over the catalyst without air and found that yield of styrene decreases in the subsequent runs. We re-oxidized the catalyst by passing air alone. Passing ethyl benzene over the activated catalyst produced styrene again. Repetition of this cycle confirms the reduction of catalyst at the EB step and its re-oxidation in the air step. We further confirmed the absence of bulk V2O5 in the (Section 3.3.) catalysts which suggests that only lattice oxygen is involved in the redox process.A further clue to the reaction mechanism was obtained by modeling of the transition state for the reaction under QST2 option of the Gaussian-09 suite. Details of QST2 option can be found elsewhere.55 The DFT molecular orbital calculations were performed using Beek's three parameter hybrid method with the Lee, Yang and Parr (B3LYP) gradient corrected correlation function.
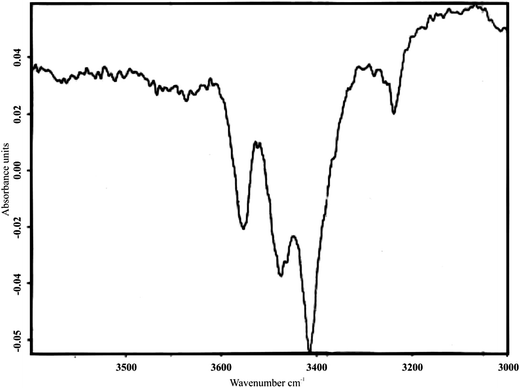
Optimized geometries of reactant model, transition state and product model are shown in Fig. 11. Formation of an hydroxyl group with one of the V sites in the catalyst is evident from Fig. 11(B). The potential energy surface diagram shown in Fig. 11(D) shows an activation energy of 114.12 kcal mol−1. The infrared spectra of the catalyst after passing ethyl benzene over the catalyst at 200 °C are reproduced in Fig. 12. The band appearing around 3400 cm−1 in Fig. 13 is attributed to the presence of V–OH group. The intensity of these bands in the fresh catalyst was very weak and can be attributed to adsorbed water molecules. Increased in the intensity of bands after treatment with ethyl benzene seems to be due to abstraction of hydrogen from ethyl benzene by the catalyst and formation of V–OH group. Thus, the DFT studies and IR recordings suggest the formation of a hydroxyl species on the surface of the catalyst. The hydroxyl species further abstracts a hydrogen atom to produce styrene and a cyclic vanadium species which catches an oxygen atom from air and regenerates the catalyst. The complete mechanism is shown schematically in Fig. 13.
 | ||
| Fig. 11 Optimized geometry of (A) reactant model (B) transition state (C) product model and (D) PES diagram for oxidative dehydrogenation of ethyl benzene over ZrV2O7 catalyst. | ||
7. Conclusions
This work demonstrates the preparation of mesoporous ZVX materials by solution combustion method and its activity towards oxidative dehydrogenation of EB to styrene using air as oxidant. Study of XRD patterns of the materials calcined at 400 °C, 600 °C and 800 °C, shows the majority of monoclinic phases of ZrO2 at higher temperature. All BET isotherms were found to be of type IV with H2 hysteresis loop typical of the mesoporous materials. The pore size of the samples obtained from BET measurements was found in the range of 3.13 to 7.7 nm. Poor intensity of Raman band at 996 cm−1 confirms almost absence of the bulk V2O5 and reveals the presence of lattice V2O5. Particle size was found in the range of 10–40 nm from TEM analysis. The electron diffraction pattern shows well crystallized ZV10 nanoparticles with no indication of any clear amorphous phase, confirming high dispersion of vanadia in ZrO2 lattice. All ZVX materials were tested for their performance towards oxidative dehydrogenation of EB taking cheap and eco-friendly air as oxidant. ZV10 was found to be the best catalyst for the reaction at 550 °C with WHSV of 2 ml h−1, EB/air ratio of 1.15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 59 (by volume). DFT optimized geometries of reactant model, transition state and product model are reported. Formation of a hydroxyl group with one of the V sites in the catalyst is concluded from this study. The potential energy surface diagram shows an activation energy of 114.12 kcal mol−1. Maximum yield of 77% of styrene with selectivity of 79% for styrene was obtained. High yield and selectivity seems to be due to moderate activation energy. The catalyst can be regenerated just by passing air for 30 minutes at 400 °C. On the basis of density function computations and FTIR spectral studies a tentative mechanism is proposed.
59 (by volume). DFT optimized geometries of reactant model, transition state and product model are reported. Formation of a hydroxyl group with one of the V sites in the catalyst is concluded from this study. The potential energy surface diagram shows an activation energy of 114.12 kcal mol−1. Maximum yield of 77% of styrene with selectivity of 79% for styrene was obtained. High yield and selectivity seems to be due to moderate activation energy. The catalyst can be regenerated just by passing air for 30 minutes at 400 °C. On the basis of density function computations and FTIR spectral studies a tentative mechanism is proposed.
Acknowledgements
Authors are thankful to Dr C. S. Gopinath, senior scientist at NCL, Pune, for a fruitful discussion on this problem. We also wish to thank the Department of Science and Technology, New Delhi, for financial support and UGC-DAE-CSR, Indore, for the XRD and Raman recordings. The group is thankful to MPCST, Bhopal, for financial assistance.References
- K. K. Kearby, in Catalysis, ed. P. H. Emmett, Reinhold, New York, 1955, vol. 3, ch. 10 Search PubMed.
- M. F. R. Pereira, J. J. M. Orfao and J. L. Figueiredo, Appl. Catal., A, 1999, 184, 153–160 CrossRef CAS.
- J. Xu, L. Wang, Y. Lui, Y. Cao, H. He and K. Fan, Catal. Lett., 2009, 133, 307–313 CrossRef CAS PubMed.
- J. P. de Celis, M. S. Villaverde, A. L. Cukierman and N. E. Amadeo, Lat. Am. Appl. Res., 2009, 39, 165–171 CAS.
- N. R. Shiju, M. Anilkumar, S. P. Mirajkar, C. S. Gopinath, B. S. Rao and C. V. Satayanarayana, J. Catal., 2005, 230, 484–492 CrossRef CAS PubMed.
- S. Kumarsrinivasan, A. Verma and C. S. Gopinath, Green Chem., 2012, 14, 461–471 RSC.
- J. Chang, D. Hong, P. Vladislav, V. Vislovskiy and S. Park, Catal. Surv. Asia, 2007, 11, 59–69 CrossRef CAS.
- F. Cavani, E. Foresti, F. Trifiro and G. Busca, J. Catal., 1987, 106, 251–262 CrossRef CAS.
- P. Forzatti, Appl. Catal., A, 2001, 222, 221–236 CrossRef CAS.
- G. Centi, Appl. Catal., A, 1996, 147, 267–298 CrossRef CAS.
- B. M. Weckhuysen and D. E. Keller, Catal. Today, 2003, 78, 25–46 CrossRef CAS.
- H. H. Kung, Adv. Catal., 1994, 40, 1–35 CAS.
- F. Cavani and F. Trifiro, Catal. Today, 1999, 51, 561–580 CrossRef CAS.
- E. A. Memedov and V. Cortes Corberan, Appl. Catal., A, 1995, 127, 1–40 CrossRef.
- T. Blasco and J. M. L. Nieto, Appl. Catal., A, 1997, 157, 117–142 CrossRef CAS.
- A. A. Lemonidou, L. Nalbandian and I. A. Vasalos, Catal. Today, 2000, 61, 333–341 CrossRef CAS.
- A. Adamski, Z. Sojka and K. Dyrek, Langmuir, 1999, 15, 5733–5741 CrossRef CAS.
- J. L. G. Fierro, L. A. Arrua, J. M. Lopez-Nieto and G. Kremenic, Appl. Catal., 1988, 37, 323–338 CrossRef CAS.
- A. Corma, J. M. Lopez-Nieto and N. Paredes, J. Mol. Catal. A: Chem., 1997, 123, 75–84 CrossRef CAS.
- U. Scharf, M. Schraml-Marth, A. Wokaun and A. Baiker, J. Chem. Soc., Faraday Trans., 1991, 3299–3307 RSC.
- I. E. Wachs, J. M. Jehng, G. Deo, B. M. Weckhuysen, V. V. Guliants, J. B. Benzigen and S. Sundaresan, J. Catal., 1997, 170, 75–88 CrossRef CAS.
- K. Routray, K. R. S. K. Reddy and G. Deo, Appl. Catal., A, 2004, 265, 103–113 CrossRef CAS PubMed.
- E. V. Kondratenko and M. Baerns, Catal. Today, 2006, 112, 60–63 CrossRef CAS PubMed.
- D. I. Enache, E. Bordes-Richard, A. Ensuque, A. Ensuque and F. Boson-Verduraz, Appl. Catal., A, 2004, 278, 93–102 CAS.
- J. M. L. Nieto, J. Soler, P. Conception, J. Herguido, M. Menendez and J. Santamaria, J. Catal., 1999, 185, 324–332 CrossRef.
- A. Khodakov, B. Olthof, A. T. Bell and E. Iglesia, J. Catal., 1999, 181, 205–216 CrossRef CAS.
- R. Sasikala, V. Sudarshan and K. S. Kulshreshtha, Eur. J. Inorg. Chem., 2006, 20, 4151–4156 CrossRef.
- P. Forzatti, E. Tronconi, A. S. Elmi and G. Busca, Appl. Catal., A, 1997, 157, 387–408 CrossRef CAS.
- Y. Nakano, T. Lizuka, H. Hattori and K. Tanabe, J. Catal., 1979, 57, 1–10 CrossRef CAS.
- A. Adamski, Z. Sojka and K. Dyrek, Langmuir, 1999, 15, 5733–5741 CrossRef CAS.
- A. Khodakov, J. Yang, S. Su, E. Iglesia and A. T. Bell, J. Catal., 1998, 177, 343–351 CrossRef CAS.
- M. De and D. Kunzru, Catal. Lett., 2005, 102, 237–246 CrossRef CAS.
- R. Sasikala, V. Sudarshan, T. Sakuntala, J. C. Sudakar, R. Naik and S. R. Bharadwaj, Appl. Catal., A, 2008, 350, 252–258 CrossRef CAS PubMed.
- D. Gazzoli, S. D. Rossi, G. Mattei, R. Spinicci and M. Valigi, J. Mol. Catal. A: Chem., 2009, 310, 17–23 CrossRef CAS PubMed.
- C. C. Hwang and T. Y. Wu, J. Mater. Sci., 2004, 39, 6111–6115 CrossRef CAS.
- J. Basak, N. Hardia, S. Saxena, R. Dixit, R. Dwivedi, S. Bhadauria and R. Prasad, Ind. Eng. Chem. Res., 2007, 46, 7039–7044 CrossRef CAS.
- A. Radheshyam, R. Dwivedi, V. S. Reddy, K. V. R. Chary and R. Prasad, Green Chem., 2002, 4, 558–561 RSC.
- G. K. Chuah and S. Jaenicke, Appl. Catal., A, 1997, 163, 261–273 CrossRef CAS.
- A. Khodakov, J. Yang, S. Su, E. Iglesia and A. T. Bell, J. Catal., 1998, 177, 343–351 CrossRef CAS.
- G. D. Sizgek, E. Sizgek, C. S. Griffith and V. Luca, Langmuir, 2008, 24, 12323–12330 CrossRef CAS PubMed.
- L. Abello, E. Husson, Y. Repelin and G. Lucazeau, Spectrochim. Acta, Part A, 1983, 39, 641–651 CrossRef.
- Y. Repelin, E. Hussan, L. Abello and G. Lucazeau, Spectrochim. Acta, Part A, 1985, 41, 993–1003 CrossRef.
- C. V. Ramana, R. J. Smith, O. M. Hussian, M. Massot and C. M. Julien, Surf. Interface Anal., 2005, 37, 406–411 CrossRef CAS.
- R. Dwivedi, A. Maurya, A. Verma, R. Prasad and K. S. Bartwal, J. Alloys Compd., 2011, 509, 6848–6851 CrossRef CAS PubMed.
- M. Kantcheva, Phys. Chem. Chem. Phys., 2000, 2, 3043–3048 RSC.
- U. L. C. Hemamala, Solid State Commun., 2007, 141, 680–684 CrossRef CAS PubMed.
- J. S. O. Evans, J. C. Hanson and A. W. Sleight, Acta Crystallogr., Sect. A: Found. Crystallogr., 1998, 54, 705–713 Search PubMed.
- H. Mohebbi, T. Ebadzadeh and F. A. Hesari, J. Power Sources, 2008, 178, 64–68 CAS.
- F. Prinetto and G. Ghiotti, J. Phys. Chem. B, 1998, 102, 10316–10325 CrossRef CAS.
- N. Kaithwas, M. Dave, S. Kar, S. Verma and K. S. Bartwal, Cryst. Res. Technol., 2010, 45, 1179–1182 CAS.
- B. M. Redddy, P. Lakshmanan, S. Loridant, Y. Yamada, T. Kobayashi, C. Lopez, T. C. Rojas and A. Fernandez, J. Phys. Chem. B, 2006, 110, 9140–9147 CrossRef PubMed.
- A. Leela Mohana Reddy and S. Ramaprabhu, J. Phys. Chem. C, 2007, 111, 7727–7734 Search PubMed.
- E. A. Mamedov and V. C. Corberan, Appl. Catal., A, 1995, 127, 1–40 CrossRef CAS.
- K. D. Chen, S. Xie, A. T. Bell and E. Iglesia, J. Catal., 2000, 195, 244–252 CrossRef CAS.
- M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, B. Mennucci, G. A. Petersson, H. Nakatsuji, M. Caricato, X. Li, H. P. Hratchian, A. F. Izmaylov, J. Bloino, G. Zheng, J. L. Sonnenberg, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, V. H. T. Nakai, J. A. Montgomery Jr, J. E. Peralta, F. Ogliaro, M. Bearpark, J. J. Heyd, E. Brothers, K. N. Kudin, V. N. Staroverov, T. Keith, R. Kobayashi, J. Normand, K. Raghavachari, A. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, N. Rega, J. M. Millam, M. Klene, J. E. Knox, J. B. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, R. E. Stratmann, O. Yazyev, A. J. Austin, R. Cammi, C. Pomelli, J. W. Ochterski, R. L. Martin, K. Morokuma, V. G. Zakrzewski, G. A. Voth, P. Salvador, J. J. Dannenberg, S. Dapprich, A. D. Daniels, O. Farkas, J. B. Foresman, J. V. Ortiz, J. Cioslowski and D. J. Fox, Gaussian 09 References, Gaussian, Inc., Wallingford CT, Revision B.01, 2010 Search PubMed.
| This journal is © The Royal Society of Chemistry 2014 |