Morphological and structural evolution from akaganeite to hematite of nanorods monitored by ex situ synchrotron X-ray powder diffraction†
Abstract
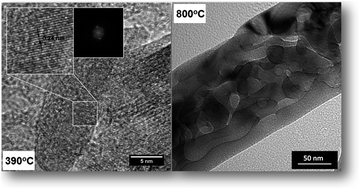
Hematite (α-Fe2O3) is one of the most abundant minerals in nature and a thermodynamically-stable phase of iron oxide. One-dimensional (1D) hematite nanostructures have attracted, due to their fundamental characteristics such as excellent chemical stability in aqueous environments and low cost, considerable interest for a series of technical applications (as sensors, catalysis, lithium ion batteries and solar energy production and storage). Our study describes the phase evolution of iron oxide nanorods, from β-FeOOH to α-Fe2O3, with increasing temperatures of thermal treatment monitored by ex situ synchrotron X-ray powder diffraction data. These data used in conjunction with the Rietveld method allowed us to infer the phase concentration, elucidate the influence of temperature on the unit cell parameters and preferred growth orientation of the nanorods. Only the use of synchrotron radiation allowed us to precisely identify and quantify the presence of a minor Fe2O3 phase, which has never before been reported for those systems. Moreover, the formation of one-dimensional nanorods was accompanied by using scanning and transmission electron microscopies (SEM and TEM). At the highest temperatures of thermal treatments the hematite nanorods exhibited changes that varied from a quasi-perfect crystal up to polycrystalline rods (>500 °C). These findings were used for discussing and explaining recent results using this kind of nanostructure synthesized under hydrothermal conditions. Changes in the preferred orientation of the crystal faces of the rods could be responsible for a low photocatalytic performance reported by this strategy.

 Please wait while we load your content...
Please wait while we load your content...