Naked-eye detection of inorganic fluoride in aqueous media using a new azo-azomethine colorimetric receptor enhanced by electron withdrawing groups†
Abstract
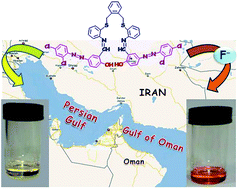
A new azo-azomethine receptor, containing active phenolic sites, has been designed and synthesized for quantitative detection and colorimetric sensing of inorganic fluoride ion in aqueous media. The introduction of four electron withdrawing groups into the backbone of the receptor makes the two phenolic groups efficient hydrogen bonding sites. Hence, the receptor is capable of competing with water molecules to detect trace amounts of inorganic fluoride ions in aqueous solutions. Accordingly, the receptor showed a remarkable colour change from light yellow to orange upon the addition of aqueous solution of NaF, enabling naked-eye F− sensing without any spectroscopic instrumentation. The recognition details of F− sensing were also evaluated using UV-Vis spectroscopy and 1H NMR titration techniques. The 1H NMR titration revealed that the colorimetric response was considered to be the direct consequence of hydrogen-bond formation between the phenolic groups of the receptor and fluoride ions followed by deprotonation. Importantly, the current sensor can detect inorganic fluoride in water even at 0.058 ppm, which is lower than the World Health Organization (WHO) permissible level. To the best of our knowledge, there have been very few examples of detection limits lower than the WHO permissible level for detecting fluoride ion in drinking water (below 1 ppm). The designed sensor has also shown highly promising results for the qualitative and quantitative detection of fluoride in real samples like sea water, toothpaste and mouthwash.

 Please wait while we load your content...
Please wait while we load your content...