The dicyclen–TPE zinc complex as a novel fluorescent ensemble for nanomolar pyrophosphate sensing in 100% aqueous solution†
Hao-Ran
Xu
,
Kun
Li
*,
Ming-Qi
Wang
,
Bo-Lin
Wang
,
Xin
Wang
and
Xiao-Qi
Yu
*
Key Laboratory of Green Chemistry and Technology, Ministry of Education, College of Chemistry, Sichuan University, Chengdu, 610064, P. R. China. E-mail: kli@scu.edu.cn; xqyu@scu.edu.cn; Fax: +86-28-85415886
First published on 29th October 2014
Abstract
A dicyclen–TPE Zn(II) complex was developed as a fluorescent ensemble for pyrophosphate (PPi) in water. The complex was characterized by X-ray single crystal diffraction, which could selectively respond to PPi over other phosphates with a large enhancement of fluorescence intensity, and the detection limit was determined to be 22.8 nM.
Pyrophosphate (PPi) plays important roles in a wide range of chemical and biological processes of human life. Therefore, the development of fluorescence probes for PPi is very essential and necessary.1 In the past few decades, a number of zinc-complex based fluorescent chemosensors have been developed.2 Several classic groups such as bis(2-pyridylmethyl) amine (DPA),3 terpyridine (tpy),4 and amide5 have been reported. Nevertheless, these probes are faced with some drawbacks: (a) low sensitivity (usually in the μM range); (b) lack of selectivity (the interference of certain nucleoside polyphosphates);6 and (c) poor water-solubility. To the best of our knowledge, there is only one example reported that showed a lower detection limit towards PPi.7 Therefore, developing water-soluble probes toward PPi possessing high selectivity and sensitivity, especially those which could respond in the nM range, is increasingly important.
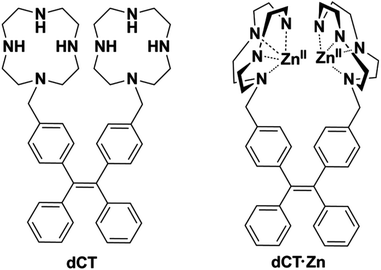
1,4,7,10-Tetraazacyclododecane (cyclen) is one kind of cyclic organic compound, which is a versatile metal chelator with excellent water-solubility,8 and its Zn(II) complexes have been developed for biological polyphosphate anion detection.9 However, their selectivity is not good and they could not discriminate PPi over other anions, especially ATP and ADP. It is possible to improve this situation by introducing a suitable fluorophore. Tetraphenyl-ethene (TPE), a kind of propeller-shaped fluorogen, could affect the fluorescence intensity according to the restriction of intramolecular rotation (RIR) mechanism.10 A number of TPE-based chemical sensors have been presented.11 It is non-emissive when the probe dissolves in solution, because energy is dissipated by the intramolecular rotation, while through aggregation or somehow else, the intramolecular rotation could be restricted. According to this mechanism, Hong and coworkers reported a DPA–TPE based sensor for PPi with a detection limit of 0.9 μM.12 Inspired by this work, we examined whether a good chemosensor for PPi could be achieved by introducing cyclen units. Herein, we present a dicyclen–TPE zinc complex (Scheme 1) and investigate its ability for biologically important phosphate anion detections in aqueous solution.
The target compound and the intermediates were successfully synthesized and characterized by NMR and ESI-MS. The conjugate (dCT) and its zinc complex (dCT·Zn) had good water solubility and suitable lipophilicity, which is in conformity with our previous design. The fluorescence response of dCT·Zn at different pH was first investigated (Fig. S1, ESI†). The results indicated that the fluorescence intensity of dCT·Zn was stable in the pH range of 2.0–13.0 and the coordination of PPi with the Zn2+ centre could result in a better enhancement of the probe fluorescence emission in the pH range of 6.0–9.0. Considering the potential biological applications of the probe, HEPES buffer (pH = 7.4) was used as the solvent for spectroscopic experiments.
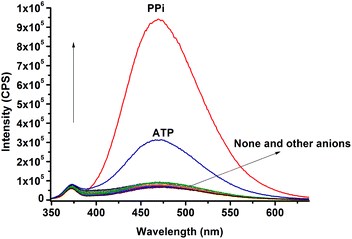
Then, the fluorescence responses of dCT to a range of metal ions were observed. As shown in Fig. S2 (ESI†), except for Cu2+ that caused a little fluorescence quenching, all other ions including Zn2+ did not cause any obvious changes in the fluorescence spectrum, which indicated that the solubility did not change significantly after the metal ions coordinate with dCT. Excellent water solubility made the benzene ring of TPE rotate freely. However, upon addition of 2.0 equiv. of Zn2+ to dCT followed by 0.5 equiv. of PPi, a 15-fold enhancement of the fluorescence intensity was observed (Fig. 1). This might be because the water solubility of the probe decreased after it coordinated with PPi, which restricted the intramolecular rotation and changed the intensity of fluorescence emission. In order to prove that it is the Zn2+ centre coordinating with PPi that leads to the enhancement, we added 0.5 equiv. of PPi to dCT and found no obvious fluorescence change. While adding another 2.0 equiv. of Zn2+ to the mixture above, a great change of the fluorescence spectrum came out again, indicating that the Zn(II)-cyclen complex could coordinate with PPi efficiently. Meanwhile, the responding abilities of dCT·Zn toward other biologically important phosphate anions and common anions were also investigated (Fig. 2). The results indicated that dCT·Zn showed good selectivity towards PPi over other anions, although ATP could result in a slight fluorescence enhancement.
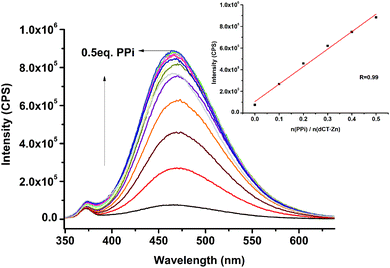 | ||
| Fig. 2 Fluorescence titration of dCT·Zn (10 μM) with PPi (0–10 eq.) in HEPES (pH = 7.4, 10 mM). The inset shows the fluorescence changes at 468 nm as a function of the amount of PPi. | ||
Due to the nucleoside existence, ATP could not form the same structure as PPi with dCT·Zn. Thus, the aggregation degree of dCT-Zn-ATP was lower than that of dCT-Zn-PPi, which leads to the weaker fluorescence emission.
A Job's plot of dCT·Zn was used to prove the 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometry with PPi (Fig. S3, ESI†). The binding constant was calculated as KdCT·Zn = 170.94 M−2 (Fig. S4, ESI†). The quantum yield of dCT·Zn increased from 0.426% to 1.757% (fluorescence of the sulphate solution as the reference, Qr = 0.560), and the detection limit of dCT·Zn was 22.8 nM. To inspect the excellent detection limit, titration of dCT·Zn with trace quantities of PPi (100 nM) was performed (Fig. S5, ESI†). A good linear relationship was achieved, which evidenced that dCT·Zn could detect PPi in the nM range. Furthermore, a kinetic study indicated that the speed of dCT·Zn interacting with PPi was fast and the enhancement of the fluorescence intensity could be stable for a long time (Fig. S6, ESI†).
1 stoichiometry with PPi (Fig. S3, ESI†). The binding constant was calculated as KdCT·Zn = 170.94 M−2 (Fig. S4, ESI†). The quantum yield of dCT·Zn increased from 0.426% to 1.757% (fluorescence of the sulphate solution as the reference, Qr = 0.560), and the detection limit of dCT·Zn was 22.8 nM. To inspect the excellent detection limit, titration of dCT·Zn with trace quantities of PPi (100 nM) was performed (Fig. S5, ESI†). A good linear relationship was achieved, which evidenced that dCT·Zn could detect PPi in the nM range. Furthermore, a kinetic study indicated that the speed of dCT·Zn interacting with PPi was fast and the enhancement of the fluorescence intensity could be stable for a long time (Fig. S6, ESI†).
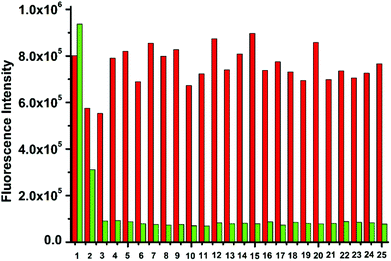
The interference experiment was conducted to determine the fluorescence emission of dCT·Zn (10 μM) containing 0.5 equivalent of P2O74−, H2PO4−, HPO42−, PO43−, Cl−, Br−, F−, I−, NO3−, Ci3−, AcO−, N3−, CN−, SO42−, SO32−, S2−, HCO3−, CO32−, ATP, ADP, AMP, Glu, Asp, oxalic acid and malonic acid (Fig. 3). It was clearly seen that dCT·Zn was highly sensitive to PPi. The emission intensity of the dCT·Zn ensemble increased upon addition of PPi; only ATP could cause a slight disturbance to a certain extent. We could also directly see a fluorescence enhancement under UV lamp illumination (Fig. S7, ESI†). Moreover, the addition of 0.5 equivalent of inorganic anions and other phosphate anions would not interfere with PPi binding to dCT·Zn (Fig. 3).
To further understand the fluorescence sensing mechanism of dCT·Zn with PPi, the absorption spectrum of the receptor was explored. The addition of Zn2+ ions to the solution of dCT did not change the ultraviolet absorption (Fig. S8, ESI†). With further addition of PPi, there were still no obvious changes in the spectrum (Fig. S9, ESI†), which confirmed that the RIR mechanism would not cause significant absorption changes.
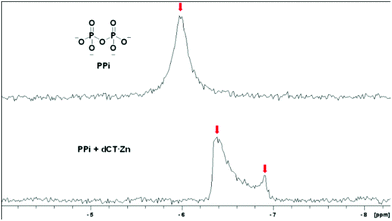
31P NMR spectroscopy was also undertaken to explain the detailed mechanism (Fig. 4). There was only one signal of 31P, suggesting that the two P atoms in PPi were equal. When PPi bound with dCT·Zn, obvious upfield shifts were observed for the 31P signal. The presence of a double 31P signal demonstrated that the two P atoms in the metal bound PPi were not magnetically equivalent, which meant the PPi bound to the binuclear zinc complex but not equally. 1H NMR titration experiments were carried out (Fig. S10, ESI†). Upon the addition of Zn2+ to the solution of dCT, the protons of methylene and ethylene groups in cyclen displayed large downfield shifts. With further addition of PPi to the dCT·Zn complex, no significant change was found. This indicated that the conformation of dCT was transformed greatly when dCT coordinated with Zn2+, but almost no further change followed PPi addition.
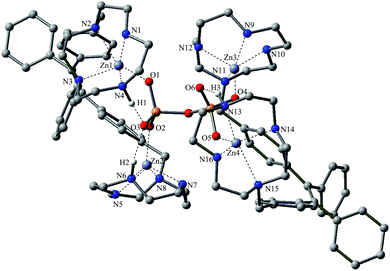
Luckily, we achieved the single crystal of dCT·Zn, and investigated it using X-ray crystallographic analysis. As shown in Fig. 5, the structure of dCT·Zn did not show complete symmetry as two Zn2+ were towards the same side. This special structure of dCT·Zn provided the possibility of a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding mode with PPi where four cyclen-Zn(II) centers towards the same side are non-covalently binding with one PPi. The details of the data collection and the structure refinement are given in the ESI (Table S1†).
1 binding mode with PPi where four cyclen-Zn(II) centers towards the same side are non-covalently binding with one PPi. The details of the data collection and the structure refinement are given in the ESI (Table S1†).
 | ||
| Fig. 5 X-ray single crystal diffraction of the dCT·Zn complex.13 | ||
To further understand the coordination properties of dCT·Zn towards PPi, DFT calculations with the B3LYP exchange functional were performed using the Gaussian 03 package. Geometries were optimized and energies were estimated at the B3LYP/6-31G(d) & LANL2DZ level. In the optimized structure of dCT·Zn·PPi (Fig. 6), two molecules of dCT·Zn coordinated with one PPi. The guest molecule is wrapped by four zinc centers, which leads to greater distortion in the geometry. These four zincs are all five-coordinated, containing four N atoms from cyclen and one O− atom from PPi. It should be noted that there are three hydrogen bonds between the PPi and dCT·Zn. The N–H in the cyclen unit serves as a hydrogen donor and the uncomplexed oxygens (O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) P) as a hydrogen receptor.
P) as a hydrogen receptor.
The formation of hydrogen bonds could provide extra stability that is favourable for the coordination. As labelled in Fig. 6, O1 could form two hydrogen bonds, while O2 only forms a single hydrogen bond, which results in the different signal of PPi in 31P NMR spectra. Meanwhile, because of the compactness of four cyclen-Zn(II) centers, ATP and other molecules could not be inserted into the cavity suitably. As all zincs in dCT·Zn toward the internal cavity, the water solubility of the whole molecule becomes much worse than before; this restricts TPE's intramolecular rotation and causes a large fluorescence intensity enhancement.
Conclusions
In summary, we have designed a simple but efficient dicyclen–TPE based fluorescent sensor, which was characterized by X-ray single crystal diffraction. dCT·Zn showed high selectivity and sensitivity towards PPi in 100% aqueous medium. The results demonstrated that dCT·Zn coordinated with PPi in a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio rather than 1
1 ratio rather than 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Notably, it is one of the few probes with the detection limit in the nm range, and is the first example of a TPE based chemosensor with single crystal structure.
1. Notably, it is one of the few probes with the detection limit in the nm range, and is the first example of a TPE based chemosensor with single crystal structure.
Acknowledgements
This work was financially supported by the National Program on Key Basic Research Project of China (973 Program, 2012CB720603 and 2013CB328900), the National Science Foundation of China (no. 21232005, 21321061, J1310008 and J1103315), and the Specialized Research Fund for the Doctoral Program of Higher Education in China (20120181130006).Notes and references
- (a) S. Anbu, S. Kamalraj, C. Jayabaskaran and P. S. Mukherjee, Inorg. Chem., 2013, 52, 8294–8296 CrossRef CAS PubMed; (b) T. Cheng, T. Wang, W. Zhu, X. Chen, Y. Yang, Y. Xu and X. Qian, Org. Lett., 2011, 13, 3656–3659 CrossRef CAS PubMed; (c) B. K. Datta, S. Mukherjee, C. Kar, A. Ramesh and G. Das, Anal. Chem., 2013, 12, 8369–8375 CrossRef PubMed; (d) N. Shao, H. Wang, X. Gao, R. Yang and W. Chan, Anal. Chem., 2010, 82, 4628–4636 CrossRef CAS PubMed.
- (a) M. Kumar, N. Kumar and V. Bhalla, Chem. Commun., 2013, 49, 877–879 RSC; (b) X. Liu, H. T. Ngo, Z. Ge, S. J. Butler and K. A. Jolliffe, Chem. Sci., 2013, 4, 1680–1686 RSC; (c) V. Luxami, K. Paul and I. H. Jeong, Dalton Trans., 2013, 42, 3783–3786 RSC; (d) Y. Mikata, A. Ugai, R. Ohnishi and H. Konno, Inorg. Chem., 2013, 52, 10223–10225 CrossRef CAS PubMed; (e) J. F. Zhang, S. Kim, J. H. Han, S.-J. Lee, T. Pradhan, Q. Y. Cao, S. J. Lee, C. Kang and J. S. Kim, Org. Lett., 2011, 13, 5294–5297 CrossRef CAS PubMed.
- (a) W.-H. Chen, Y. Xing and Y. Pang, Org. Lett., 2011, 13, 1362–1365 CrossRef CAS PubMed; (b) P. Das, N. B. Chandar, S. Chourey, H. Agarwalla, B. Ganguly and A. Das, Inorg. Chem., 2013, 52, 11034–11041 CrossRef CAS PubMed; (c) F. Huang and G. Feng, RSC Adv., 2014, 4, 484–487 RSC; (d) A. S. Rao, S. Singha, W. Choi and K. H. Ahn, Org. Biomol. Chem., 2012, 10, 8410–8417 RSC.
- L.-J. Liang, X.-J. Zhao and C.-Z. Huang, Analyst, 2012, 137, 953–958 RSC.
- (a) G. Ambrosi, M. Formica, V. Fusi, L. Giorgi, A. Guerri, E. Macedi, M. Micheloni, P. Paoli, R. Pontellini and P. Rossi, Inorg. Chem., 2009, 48, 5901–5912 CrossRef CAS PubMed; (b) X.-H. Huang, Y. Lu, Y.-B. He and Z.-H. Chen, Eur. J. Org. Chem., 2010, 1921–1927 CrossRef; (c) R. K. Pathak, V. K. Hinge, A. Rai, D. Panda and C. P. Rao, Inorg. Chem., 2011, 51, 4994–5005 CrossRef PubMed; (d) P. Sokkalingam, D. S. Kim, H. Hwang, J. L. Sessler and C.-H. Lee, Chem. Sci., 2012, 3, 1819–1824 RSC; (e) M. Zhang, W.-J. Ma, C.-T. He, L. Jiang and T.-B. Lu, Inorg. Chem., 2013, 52, 4873–4879 CrossRef CAS PubMed.
- (a) J. Deng, P. Yu, L. Yang and L. Mao, Anal. Chem., 2013, 85, 2516–2522 CrossRef CAS PubMed; (b) K. Ghosh, A. R. Sarkar, A. Samadder and A. R. Khuda-Bukhsh, Org. Lett., 2012, 14, 4314–4317 CrossRef CAS PubMed; (c) S. Khatua, S. H. Choi, J. Lee, K. Kim, Y. Do and D. G. Churchill, Inorg. Chem., 2009, 48, 2993–2999 CrossRef CAS PubMed; (d) S. Kim, M. S. Eom, S. K. Kim, S. H. Seo and M. S. Han, Chem. Commun., 2013, 49, 152–154 RSC; (e) R. K. Pathak, K. Tabbasum, A. Rai, D. Panda and C. P. Rao, Anal. Chem., 2012, 84, 5117–5123 CrossRef CAS PubMed; (f) I. Ravikumar and P. Ghosh, Inorg. Chem., 2011, 50, 4229–4231 CrossRef CAS PubMed; (g) O. G. Tsay, S. T. Manjare, H. Kim, K. M. Lee, Y. S. Lee and D. G. Churchill, Inorg. Chem., 2013, 52, 10052–10061 CrossRef CAS PubMed.
- S. Bhowmik, B. N. Ghosh, V. Marjomäki and K. Rissanen, J. Am. Chem. Soc., 2014, 136, 5543–5546 CrossRef CAS PubMed.
- H.-R. Xu, K. Li, Q. Liu, T.-M. Wu, M.-Q. Wang, J.-T. Hou, Z. Huang, Y.-M. Xie and X.-Q. Yu, Analyst, 2013, 138, 2329–2334 RSC.
- (a) Z. Zeng, A. A. J. Torriero, A. M. Bond and L. Spiccia, Chem. – Eur. J., 2010, 16, 9154–9163 CrossRef CAS PubMed; (b) F. Schmidt, S. Stadlbauer and B. König, Dalton Trans., 2010, 39, 7250–7261 RSC.
- (a) C. W. T. Leung, Y. Hong, J. Hanske, E. Zhao, S. Chen, E. V. Pletneva and B. Z. Tang, Anal. Chem., 2014, 86, 1263–1268 CrossRef CAS PubMed; (b) Y. Yuan, R. T. K. Kwok, G. Feng, J. Liang, J. Geng, B. Z. Tang and B. Liu, Chem. Commun., 2014, 50, 295–297 RSC.
- (a) H. Shi, N. Zhao, D. Ding, J. Liang, B. Z. Tang and B. Liu, Org. Biomol. Chem., 2013, 11, 7289–7296 RSC; (b) Y. Hong, M. Häubler, J. W. Y. Lam, Z. Li, K. K. Sin, Y. Dong, H. Tong, J. Liu, A. Qin, R. Renneberg and B. Z. Tang, Chem. – Eur. J., 2008, 14, 6428–6437 CrossRef CAS PubMed; (c) Y. Hong, S. Chen, C. W. T. Leung, J. W. Y. Lam and B. Z. Tang, Chem. – Asian J., 2013, 8, 1806–1812 CrossRef CAS PubMed; (d) Y. Hong, H. Xiong, J. W. Y. Lam, M. Häubler, J. Liu, Y. Yu, Y. Zhong, H. H. Y. Sung, I. D. Williams, K. S. Wong and B. Z. Tang, Chem. – Eur. J., 2010, 16, 1232–1245 CrossRef CAS PubMed; (e) H. Shi, R. T. K. Kwok, J. Liu, B. Xing, B. Z. Tang and B. Liu, J. Am. Chem. Soc., 2012, 134, 17972–17981 CrossRef CAS PubMed; (f) X. Chen, X. Y. Shen, E. Guan, Y. Liu, A. Qin, J. Z. Sun and B. Z. Tang, Chem. Commun., 2013, 49, 1503–1505 RSC; (g) T. Tian, X. Chen, H. Li, Y. Wang, L. Guo and L. Jiang, Analyst, 2013, 138, 991–9944 RSC.
- C. Park and J.-I. Hong, Tetrahedron Lett., 2010, 51, 1960–1962 CrossRef CAS PubMed.
- CCDC 998114 contains the X-ray data for the compound.
Footnote |
| † Electronic supplementary information (ESI) available: General experimental procedures and spectroscopic data (1H-NMR, 13C-NMR and HRMS) for the corresponding products. CCDC 998114. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c4qo00243a |
| This journal is © the Partner Organisations 2014 |