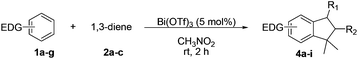Bi(OTf)3-catalysed synthesis of substituted indanes by a double hydroarylation of unactivated 1,3-dienes†‡
Bastien
Cacciuttolo
,
Pierrick
Ondet
,
Sophie
Poulain-Martini
,
Gilles
Lemière
and
Elisabet
Duñach
*
Institut de Chimie de Nice UMR 7272, Université de Nice Sophia-Antipolis, CNRS. Parc Valrose, 06108 Nice Cedex 2, France. E-mail: dunach@unice.fr; Fax: (+) 33492076151
First published on 10th July 2014
Abstract
The intermolecular reaction between differently substituted electron-rich arenes and unactivated 1,3-dienes catalysed by bismuth(III) triflate is presented. This highly atom-economical process is conducted under very mild conditions and leads efficiently to substituted indane derivatives through a tandem bis-hydroarylation.
The Friedel–Crafts reaction remains a process of choice for the functionalisation of aromatic compounds through carbon–carbon bond formation.1 Over the last century, several Lewis and Brønsted acids have been used to perform Friedel–Crafts reactions in the presence of alkyl halides. Despite the importance of this transformation, it may present some drawbacks, such as the use of toxic halide derivatives, the requirement of over-stoichiometric amounts of Lewis acids and therefore the production of non-negligible amounts of waste.
The hydroarylation reaction represents the most atom-economical way to functionalize aromatic nuclei, since no by-products are formed. Therefore, this process appears as a sustainable alternative to the original Friedel–Crafts reaction. The main reports on hydroarylation of olefins were devoted to the addition of aromatic compounds to vinylarenes,1e typically styrene derivatives. The extension to conjugated dienes has been scarcely reported, possibly because of the increased difficulties to control the selectivities, avoiding polyfunctionalisation and diene polymerisation. The achievements made with 1,3-dienes in this field were mainly the synthesis of chromanes by reaction of isoprene with phenol derivatives.2 However, the direct allylation of non-phenolic arenes using 1,3-dienes is rare and, except for some sporadic reports,3 it usually offers limited results in terms of yields and selectivities.4
Recently, our group described a mild and efficient intramolecular hydroarylation of olefins5 and allenes6 catalysed by relatively non-expensive and easily available Bi(OTf)3,7 giving rise to interesting aromatic carbobicycles. This prompted us to study the more challenging Lewis acid-catalysed intermolecular reaction between 1,3-dienes and aromatic compounds, aiming at a straightforward access to diversely polysubstituted indanes through a tandem allylation/cyclisation process. Some related studies have been attempted using a large excess of sulfuric acid as the catalyst, however with limited success.8 We herein report results concerning the catalytic, efficient and particularly atom-economical synthesis of indanes carried out under mild conditions.
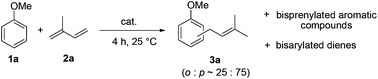
We initiated our study by examining the coupling of anisole 1a and isoprene 2a in the presence of various metal triflate catalysts. We rapidly grasped the difficulty of achieving this intermolecular reaction with a good selectivity in favour of a single coupling product. Monoprenylated anisole 3a was usually obtained as a mixture of para and ortho regioisomers in a 75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25 ratio. A slight double bond isomerisation was also observed. Besides this desired monocoupling compound, anisole polyprenylation, diene polyarylation and polymer formation were competitive reactions occurring concomitantly, as observed by GC-MS analysis.
25 ratio. A slight double bond isomerisation was also observed. Besides this desired monocoupling compound, anisole polyprenylation, diene polyarylation and polymer formation were competitive reactions occurring concomitantly, as observed by GC-MS analysis.
Optimization of the reaction conditions has been carried out in order to limit these undesired side-reactions. Results are summarized in Table 1. The screening of catalysts (10 mol%) in nitromethane (Table 1, entries 1–10) highlighted Sn(OTf)2 and Bi(OTf)3 as promising intermolecular hydroarylation candidates (Table 1, entries 4 and 8). The reaction proved to be less efficient in dichloroethane and dichloromethane as compared to nitromethane which is a low-coordinating polar solvent favouring the stabilisation of cationic intermediates (Table 1, entries 8, 11 and 12). To limit the diene polymerisation and polyarylation, anisole was used in excess (entries 13–16). A 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio of anisole to isoprene without an additional solvent allowed to increase the selectivity in favour of 3a with Sn(OTf)2 and Bi(OTf)3 to 62% and 53%, respectively (entries 15 and 16). Lowering the Sn(OTf)2 loading to 1–5 mol% had a negative effect on the conversion (entries 17 and 18). Remarkably, the yield of 3a could be increased to an excellent yield of 94% by performing the reaction with only 1 mol% of Bi(OTf)3 indicating its higher catalytic activity (entry 20).
1 ratio of anisole to isoprene without an additional solvent allowed to increase the selectivity in favour of 3a with Sn(OTf)2 and Bi(OTf)3 to 62% and 53%, respectively (entries 15 and 16). Lowering the Sn(OTf)2 loading to 1–5 mol% had a negative effect on the conversion (entries 17 and 18). Remarkably, the yield of 3a could be increased to an excellent yield of 94% by performing the reaction with only 1 mol% of Bi(OTf)3 indicating its higher catalytic activity (entry 20).
| Entrya | Cat. (mol%) | Ratio 1a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2a 2a |
Solvent | Yield of 3ab |
|---|---|---|---|---|
| a To a solution of anisole 1a in the solvent (0.5 M), isoprene 2a was added followed by M(OTf)n (1–10 mol%) at room temperature. The solution was stirred for 4 h and monitored by GC. b GC yields using dodecane as the internal standard. c The reaction was conducted at 40 °C. d The reaction was conducted at 10 °C. | ||||
| 1 | In(OTf)3 (10) | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
CH3NO2 | 6% |
| 2 | Cu(OTf)2 (10) | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
CH3NO2 | 5% |
| 3 | Al(OTf)3 (10) | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
CH3NO2 | 4% |
| 4 | Bi(OTf)3 (10) | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
CH3NO2 | 32% |
| 5 | AgOTf (10) | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
CH3NO2 | — |
| 6 | Zn(OTf)2 (10) | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
CH3NO2 | 5% |
| 7 | Yb(OTf)3 (10) | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
CH3NO2 | — |
| 8 | Sn(OTf)2 (10) | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
CH3NO2 | 49% |
| 9 | Sn(OTf)4 (10) | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
CH3NO2 | 3% |
| 10 | Sc(OTf)3 (10) | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
CH3NO2 | 12% |
| 11 | Sn(OTf)2 (10) | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
(CH2Cl)2 | 22% |
| 12 | Sn(OTf)2 (10) | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
CH2Cl2 | 10% |
| 13 | Sn(OTf)2 (10) |
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1 1
|
— | 31% |
| 14 | Sn(OTf)2 (10) |
5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1 1
|
— | 45% |
| 15 | Sn(OTf)2 (10) |
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1 1
|
— | 62% |
| 16 | Bi(OTf)3 (10) |
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1 1
|
— | 53% |
| 17 | Sn(OTf)2 (5) | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
— | 15% |
| 18 | Sn(OTf)2 (1) | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
— | 15% |
| 19 | Bi(OTf)3 (5) | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
— | 55% |
| 20 | Bi(OTf)3 (1) |
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1 1
|
— | 94% |
| 21c | Bi(OTf)3 (1) | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
— | 52% |
| 22d | Bi(OTf)3 (1) | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
— | 24% |
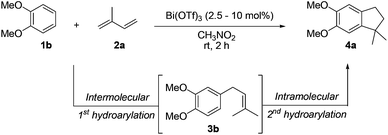
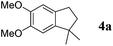
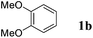
On the other hand, Bi(OTf)3 has been reported as a good catalyst for the intramolecular hydroarylation of olefins. Being interested in performing two hydroarylations in one step, we turned our attention to the coupling of isoprene 2a with more electron-rich arenes. A better control of the regioselectivity and a favoured cyclisation process were expected. In the presence of 5 mol% of Bi(OTf)3 and a slow addition (over 1 hour) of isoprene 2a, 1,2-dimethoxybenzene 1b reacted at room temperature in nitromethane (the initial concentration of the arene: 0.25 mol L−1) to afford the desired indane 4a as a single regioisomer with 54% yield. The other products detected by GC-MS analysis correspond to a mixture of aromatic derivatives with two and three isoprene units (Table 2, entry 1). Surprisingly, the same reaction carried out at a higher initial arene concentration enabled limitation of the aromatic polyfunctionalisation and improved the yield of indane 4a to 79% (Table 2, entry 3).
| Entrya | Catalyst loading (mol%) | Initial conc. of 1b (mol L−1) | Yield of 4ab (%) |
|---|---|---|---|
| a General procedure: to a solution of 1,2-dimethoxybenzene (0.25–1 mmol) and Bi(OTf)3 (2.5–10 mol%) in CH3NO2 (1 mL) was added isoprene (1.5 mmol) in CH3NO2 (1 mL) over 1 hour at rt and the solution was further stirred for 1 h. b Isolated yields. c The reaction was conducted at 40 °C. | |||
| 1 | 5 | 0.25 | 54 |
| 2 | 5 | 0.5 | 61 |
| 3 | 5 | 1 | 79 |
| 4 | 2.5 | 1 | 42 |
| 5 | 10 | 1 | 33 |
| 6c | 5 | 1 | 44 |
Further variations of the reaction parameters such as the catalyst loading and the temperature were carried out without enhancement of the yield (Table 2, entries 4–6).
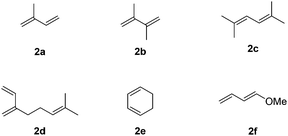
We then extended this novel catalytic sequential transformation to the synthesis of other indane derivatives with a variation of the arene and diene partners. Six commercially available and variously substituted dienes were tested (Fig. 1).
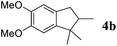
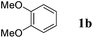
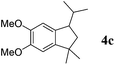
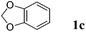
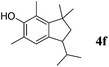
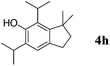
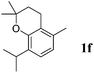
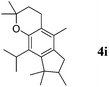
Like isoprene 2a, 2,3-dimethyl-1,3-butadiene 2b reacted efficiently under the same conditions (as in Table 2, entry 3) with 1,2-dimethoxybenzene 1b to afford the desired indane product 4b with an excellent yield of 95% (Table 3, entry 2). Interestingly, the same arene efficiently coupled as well with a more hindered 1,3-diene such as 2,5-dimethyl-2,4-hexadiene 2c (Table 3, entry 3). In this case, the substituted indane 4c was obtained with 71% yield and a total control of the regioselectivity through the tandem allylation/cyclisation process. It is worth mentioning that the other 1,3-dienes tested were not effective in this reaction. Myrcene 2d was prone to polymerisation and cyclohexadiene 2e afforded a mixture of polyallylated compounds. In addition, no coupling products could be detected with (E)-1-methoxybuta-1,3-diene 2f. Therefore, we turned our attention to the coupling of dienes 2a–c with other electron-rich aromatic derivatives. 1,3-Benzodioxole 1c reacted cleanly with isoprene 2a to lead to the polycyclic compound 4d with 91% yield (entry 4). Under the same conditions, less activated anisole 1a was found to react selectively with the tetrasubstituted 1,3-diene 2c at room temperature (entry 5). The lower polymerisation ability due to the higher hindrance of this diene in comparison with isoprene 2a allowed the regioselective monofunctionalisation of anisole at the para position. The increase of the temperature after total consumption of the starting diene 2c afforded the cyclised product 4e in 79% yield. 2,6-Dimethylphenol 1d and more hindered 2,6-diisopropylphenol 1e efficiently reacted regioselectively with 2,5-dimethyl-2,4-hexadiene 2c to afford substituted indanes 4f and 4g, respectively, with excellent yields (entries 6 and 7). Isoprene 2a could also be coupled to the phenol derivative 1e giving rise to indane 4h, known as the artificial musk HDDI, which possesses a very strong musk odour (entry 8).9 This catalytic procedure represents an efficient access to this artificial fragrance compound under very mild reaction conditions. Fully substituted aromatic compound 4i was also selectively obtained by reaction of 2,3-dimethylbuta-1,3-diene 2b and the corresponding chromane derivative 1f with a nearly quantitative yield (entry 9).
| Entrya | Arene | Diene | Product | Yieldb |
|---|---|---|---|---|
| a General procedure: to a solution of arene (1 mmol) and Bi(OTf)3 (0.05 mmol) in CH3NO2 (1 mL) was added the diene (1.5 mmol) in CH3NO2 over 1 h at room temperature. The reaction was further stirred at rt for 1 h. b Isolated yields. c The temperature was increased to reflux after the total consumption of the diene (GC monitoring). | ||||
| 1 |
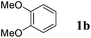
|
2a |
|
79% |
| 2 |
|
2b |
|
95% |
| 3 |
|
2c |
|
71% |
| 4 |
|
2a |
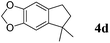
|
91% |
| 5 |
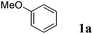
|
2c |
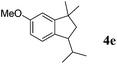
|
79%c |
| 6 |
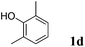
|
2c |
|
93%c |
| 7 |
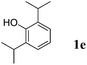
|
2c |
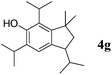
|
94% |
| 8 |
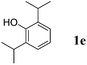
|
2a |
|
82% |
| 9 |
|
2b |
|
97%c |
As an extension of this methodology, we were able to perform an efficient double tandem allylation/cyclization by the reaction of thymol 1g with two isoprene units 2a and a catalytic amount of Bi(OTf)3 (Scheme 1). The first isoprene unit reacted with the phenolic part to form a chromane sub-structure. The second equivalent gave a regioselective access to the fully substituted benzene 4j with an excellent total yield of 92%. This unprecedented transformation, involving four bond formation steps in a one-pot reaction, was very efficient at room temperature in dichloroethane with heating at 50 °C after the diene addition at room temperature, to facilitate the final intramolecular hydroarylation.
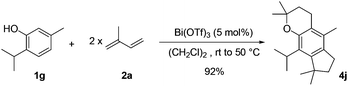 | ||
| Scheme 1 Efficient synthesis of tricyclic compound 4j from commercially available thymol and isoprene. | ||
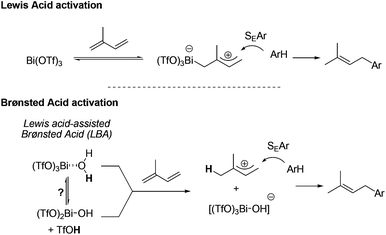
From a mechanistic point of view, two activation modes of the 1,3-diene can be suggested (Scheme 2). The first pathway invokes the formation of an allylic cation generated by the direct activation of the diene by Bi(OTf)3. Trapping of this cationic intermediate by the aromatic derivative through an SEAr type reaction furnishes the allylated arene. This possibility has already been proposed for the Bi(OTf)3-catalysed hydroamination of 1,3-dienes.10 The alternative pathway involves a Brønsted acid catalysis. Even though the first Lewis acid activation pathway cannot be entirely ruled out, the experimentally observed activation of highly hindered diene 2c suggests that a Brønsted-type acid catalysis could be preferred. In this case, the acid species would be produced either by hydrolysis or by hydration of the triflate salt. The first possibility would liberate triflic acid whereas the latter one would form an acidic hydrated metal which could act as a Lewis acid-assisted Brønsted acid (LBA)-type catalyst.11 The activity of triflate salts is still a matter of debate12 but theoretical calculations tend to suggest that hydration of triflate and trifimidate salts is much favored over their hydrolysis.13 Finally, the second catalysed intramolecular hydroarylation5 gives rise to the indane products.
In conclusion, we have developed an efficient Bi(III)-catalysed regioselective condensation of 1,3-dienes with arenes. This highly atom-economical process generally using 5 mol% of the catalyst leads to substituted indane derivatives through a sequential intermolecular hydroarylation of the diene followed by an intramolecular hydroarylation of the resulting tethered olefin. The control of the reaction parameters such as the addition rate, the solvent, the concentration of the substrate and the reaction temperature has to be finely tuned, in order to limit side-reactions and diene polymerisation. Moreover, the regioselectivity of these transformations is excellent and can be predicted by the analysis of the electronic and steric contributions of the different groups present on the starting arene.
Acknowledgements
The French project ANR is gratefully acknowledged (grant number ANR-2013-ALEA-009-01).Notes and references
- (a) M. Bandini, A. Melloni and A. Umani-Ronchi, Angew. Chem., Int. Ed., 2004, 43, 550–556 CrossRef CAS PubMed; (b) M. Bandini, E. Emer, S. Tommasi and A. Umani-Ronchi, Eur. J. Org. Chem., 2006, 3527–3544 CrossRef CAS; (c) T. B. Poulsen and K. A. Jorgensen, Chem. Rev., 2008, 108, 2903–2915 CrossRef CAS PubMed; (d) S.-L. You, Q. Cai and M. Zeng, Chem. Soc. Rev., 2009, 38, 2190–2201 RSC; (e) M. Rueping and B. J. Nachtsheim, Beilstein J. Org. Chem., 2010, 6, 6 Search PubMed; (f) V. Terrasson, F. R. Marcia and J. M. Campagne, Eur. J. Org. Chem., 2010, 2635–2655 CrossRef CAS; (g) M. Zeng and S.-L. You, Synlett, 2010, 1289–1301 CAS.
- (a) S. W. Youn and J. I. Eom, J. Org. Chem., 2006, 71, 6705–6707 CrossRef CAS PubMed; (b) L. A. Adrio and K. K. Hii, Chem. Commun., 2008, 2325–2327 RSC.
- (a) M.-C. P. Yeh, M.-N. Lin, Y.-S. Chou, T.-C. Lin and L.-Y. Tseng, J. Org. Chem., 2011, 76, 4027–4033 CrossRef CAS PubMed; (b) M. Niggemann and N. Bisek, Chem. – Eur. J., 2010, 16, 11246–11249 CrossRef CAS PubMed; (c) M.-Z. Wang, M.-K. Wong and C.-M. Che, Chem. – Eur. J., 2008, 14, 8353–8364 CrossRef CAS PubMed; (d) Y. Kuninobu, T. Matsuki and K. Takai, J. Am. Chem. Soc., 2009, 131, 9914–9915 CrossRef CAS PubMed; (e) K. E. Judd and L. Caggiano, Org. Biomol. Chem., 2011, 9, 5201–5210 RSC.
- (a) C. Cativiela, J. García, M. García-Matres, J. A. Mayoral, F. Figueras, J. Fraile, T. Cseri and B. Chiche, Appl. Catal., A, 1995, 123, 273–287 CrossRef CAS; (b) V. N. Ipatieff, H. Pines and R. E. Schaad, J. Am. Chem. Soc., 1944, 66, 816–817 CrossRef CAS; (c) H. Pines, B. Kvetinskas, J. A. Vesely and E. Baclawski, J. Am. Chem. Soc., 1951, 73, 5173–5175 CrossRef CAS; (d) W. Proell, J. Org. Chem., 1951, 16, 178–184 CrossRef CAS.
- B. Cacciuttolo, S. Poulain-Martini and E. Duñach, Eur. J. Org. Chem., 2011, 3710–3714 CrossRef CAS.
- G. Lemière, B. Cacciuttolo, E. Belhassen and E. Duñach, Org. Lett., 2012, 14, 2750–2753 CrossRef PubMed.
- For reviews on bismuth catalysis: (a) J. M. Bothwell, S. W. Krabbe and R. S. Mohan, Chem. Soc. Rev., 2011, 40, 4649–4707 RSC; (b) H. Gaspard-Iloughmane and C. Le Roux, Eur. J. Org. Chem., 2004, 2517–2532 CrossRef CAS; (c) T. Ollevier, Org. Biomol. Chem., 2013, 11, 2740–2755 RSC. For recent reviews on the use of bismuth(III) triflate in catalysis: (d) X. Li, X. Yang, H. Chang, Y. Li, B. Ni and W. Wei, Eur. J. Org. Chem., 2011, 3122–3125 CrossRef; (e) K. Komeyama, N. Saigo, M. Miyagi and K. Takaki, Angew. Chem., Int. Ed., 2009, 48, 9875–9878 CrossRef CAS PubMed; (f) B. D. Kelly, J. M. Allen, R. E. Tundel and T. H. Lambert, Org. Lett., 2009, 11, 1381–1383 CrossRef CAS PubMed; (g) O. A. Attanasi, F. Gianfranco, G. Giorgi, F. Mantellini, V. Karapetyan and P. Langer, Tetrahedron, 2009, 65, 5456–5461 CrossRef CAS PubMed; (h) P. Rubenbauer, E. Herdtweck, T. Strassner and T. Bach, Angew. Chem., Int. Ed., 2008, 47, 10106–10109 CrossRef CAS PubMed; (i) K. Komeyama, K. Takahashi and K. Takaki, Org. Lett., 2008, 10, 5119–5122 CrossRef CAS PubMed; (j) M. Rueping, B. J. Nachtsheim and T. Scheidt, Org. Lett., 2006, 8, 3717–3719 CrossRef CAS PubMed; (k) M. Rueping, B. J. Nachtsheim and W. Ieawsuwan, Adv. Synth. Catal., 2006, 348, 1033–1037 CrossRef CAS; (l) F. Mühlthau, O. Schuster and T. Bach, J. Am. Chem. Soc., 2005, 127, 9348–9349 CrossRef PubMed.
- (a) T. F. Wood and J. Angiolini, Tetrahedron Lett., 1963, 4, 1–8 CrossRef; (b) E. J. Eisenbraun, W. M. Harms, V. A. Palaniswamy, H. H. Chen, P. J. Porcaro, T. F. Wood and M. Chien, J. Org. Chem., 1982, 47, 342–346 CrossRef CAS; (c) T. F. Wood, U. S. Patent, 3078319, 1963 Search PubMed.
- T. F. Wood and G. H. Goodwin, GE Patent, 1801662, 1977 Search PubMed.
- H. Qin, N. Yamagiwa, S. Matsunaga and M. Shibasaki, J. Am. Chem. Soc., 2009, 128, 1611–1614 CrossRef PubMed.
- (a) H. Yamamoto and K. Futatsugi, Angew. Chem., Int. Ed., 2005, 44, 1924–1942 CrossRef CAS PubMed; (b) L. Coulombel, M. Rajzmann, J.-M. Pons, S. Olivero and E. Duñach, Chem. – Eur. J., 2006, 12, 6356–6365 CrossRef CAS PubMed; (c) D. B. G. Williams and M. Lawton, J. Mol. Catal. A: Chem., 2010, 317, 68–71 CrossRef CAS PubMed; (d) C. H. Cheon, O. Kanno and F. D. Toste, J. Am. Chem. Soc., 2011, 133, 13248–13251 CrossRef CAS PubMed; (e) O. Kanno, W. Kuriyama, Z. J. Wang and F. D. Toste, Angew. Chem., Int. Ed., 2011, 50, 9919–9922 CrossRef CAS PubMed.
- (a) S. Kobayashi, S. Nagayama and T. Busujima, J. Am. Chem. Soc., 1998, 120, 8287–8288 CrossRef CAS; (b) T. C. Wabnitz, J.-Q. Yu and J. B. Spencer, Chem. – Eur. J., 2004, 10, 484–493 CrossRef CAS PubMed; (c) D. B. G. Williams and M. Lawton, J. Mol. Catal. A: Chem., 2010, 317, 68–71 CrossRef CAS PubMed; (d) A. Dzudza and T. J. Marks, Chem. – Eur. J., 2010, 16, 3403–3422 CrossRef CAS PubMed; (e) D. C. Rosenfeld, S. Shekhar, A. Takemiya, M. Utsunomiya and J. F. Hartwig, Org. Lett., 2006, 8, 4179–4182 CrossRef CAS PubMed; R. F. Lambert, R. J. Hinkle, S. E. Ammann, Y. Lian, J. Liu, S. E. Lewis and R. D. Pike, J. Org. Chem., 2011, 76, 9269–9277 Search PubMed.
- (a) J. Godeau, F. Fontaine-Vive, S. Antoniotti and E. Dunach, Chem. – Eur. J., 2012, 18, 16815–16822 CrossRef CAS PubMed; (b) P. Nava, Y. Carissan, J. Drujon, F. Grau, J. Godeau, S. Antoniotti, E. Duñach and S. Humbel, ChemCatChem, 2014, 6, 500–507 CrossRef CAS.
Footnotes |
| † This work is dedicated to Professor Max Malacria on the occasion of his 65th birthday. |
| ‡ Electronic supplementary information (ESI) available. See DOI: 10.1039/c4qo00149d |
| This journal is © the Partner Organisations 2014 |