Enantioselective heterogeneous Brønsted acid catalysis
Anil Kumar
Mutyala
and
Nitin T.
Patil
*
Division of Organic Chemistry, CSIR – National Chemical Laboratory, Dr. Homi Bhabha Road, Pune 411 008, India. E-mail: n.patil@ncl.res.in
First published on 25th March 2014
Abstract
This highlight describes the synthesis and catalytic activities of heterogeneous chiral Brønsted acid catalysts. The heterogeneous catalysts are stable, easily separable from the reaction mixture and can be used multiple times without any loss of activity. As a remarkable particularity, the use of heterogeneous catalyst systems has been exemplified for designing continuous flow reactors.
Introduction
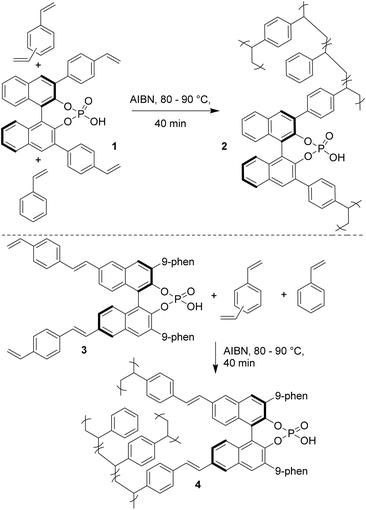
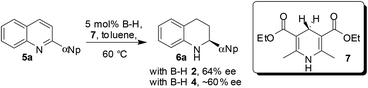
Chiral BINOL-derived phosphonic acid catalysis is a very active area of research.1 Various enantioselective reactions are published every week, amazing reactivities and selectivities are often observed, and the number of applications in total synthesis is increasing. Clearly, success in enantioselective Brønsted acid catalysis is undisputed. However, the main drawback associated with this chemistry is that the catalysts are difficult to synthesize, highly expensive and of high molecular weights and therefore even at low catalyst loadings several milligrams of catalyst are needed to be used to perform small scale reactions. In addition, their recovery is difficult since those catalysts are soluble in organic solvents. Hence, it is a great challenge for synthetic organic chemists to develop heterogeneous Brønsted acid catalysts, by immobilizing catalysts onto polymeric supports, to perform the reactions. Such heterogeneous catalysis will be extremely useful since the starting materials can be easily converted into products and the catalyst can simply be filtered off for further use.2 Though, the problem of the high molecular weight would still exist/intensify in this type of polymer immobilized chiral Brønsted acid organocatalyst, the fact that they can be easily recovered might compensate the drawbacks associated with their notoriously tedious synthesis. Surprisingly, until recently, there were no reports on the heterogenization of the Brønsted acid catalysts presumably due to the misconception that polymer-bound catalysts suffer from lower catalytic activity and enantioselection compared to their homogeneous counterparts.In 2010, a research group of Rueping in collaboration with Sugiono reported the first example of immobilization of a chiral Brønsted acid onto a polymer network.3 The polymer-bound Brønsted acid catalyst can not only be easily recovered from the reaction mixture but it can also be easily reused in several catalytic cycles without any loss of reactivity and selectivity. The heterogeneous catalysts 2 and 4 were prepared by cross-linking radical polymerization with styrene and divinylbenzene from catalysts 1 and 3, respectively (Scheme 1). The catalytic ability of the polymer-supported catalysts 2 and 4 was subsequently evaluated in transfer hydrogenations of quinolines (5a→6a) and benzoxazines (8→9) as depicted in Schemes 2 and 3, respectively. It was found that the catalytic activities and the asymmetric induction are comparable to those of the homogeneous reactions demonstrating the efficiency of this new catalyst system. It is noteworthy that the catalysts 2 and 4 are in the form of polymer-stick and therefore after completion of the reaction the separation of the catalyst can be simply achieved by pulling the stick from the reaction mixture. The polymer stick was successfully recycled and reused for 12 cycles without any loss of activity (Scheme 3).
In general, the bulky nature of the substituents at the 3,3′ position of BINOL derived phosphoric acid is crucial for obtaining high enantioselectivity.1 This reason could be attributed to the increase in rigidity of the catalyst structure which in turn develops a rigid chiral pocket which is necessary for enantioselective induction. The heterogeneous catalysts 2 and 4 developed by Rueping/Sugiono also bear bulky substituents at the 3,3′ position. In the case of catalyst 2, the polymer is providing the steric hindrance; whereas, in the case of catalyst 4 the steric hindrance is already embedded in the monomer and the polymer chain is located far away from the active sites.
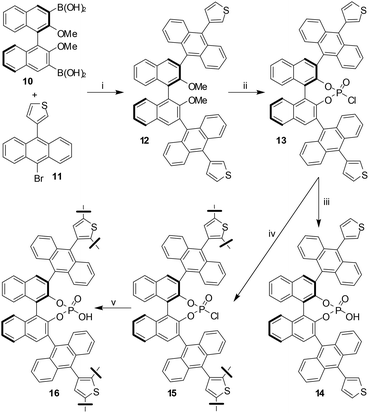
Very recently, collaborative efforts of the research group of Thomas and Blechert revealed a new chiral microporous recyclable heterogeneous catalyst made from a BINOL-derived phosphoric acid (BNPPA).4 The first step in preparation of the catalyst was the Suzuki coupling reaction between diboronic acid 10 and 3-(10-bromoanthracen-9-yl)-thiophene 11 to give 12 in good yield. Subsequent demethylation with BBr3 followed by treatment with POCl3 gave BNPPA chloride 13 which on hydrolysis with aq. HCl afforded the desired product 14. However, this monomer 14 turned out not to be suitable for oxidative coupling owing to solubility issues. Incidentally, the FeCl3-mediated oxidative polymerization reaction was successful with the highly soluble 13 affording the polymeric acid chloride 15. Hydrolysis of 15 with aq. HCl and multiple washing with ethanol, THF, and CHCl3 gave the desired product 16 as a powder, which was found to be insoluble in solvents such as EtOH, THF, CHCl3 and CH2Cl2 (Scheme 4).
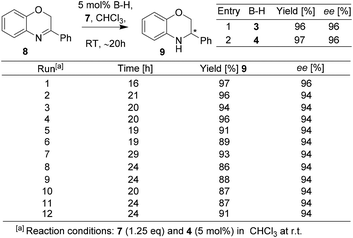
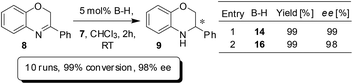
The BNPPA derived heterogeneous catalyst with high permanent surface area was found to be highly active and selective in enantioselective organocatalysis. For instance, transfer hydrogenation of 3-phenyl-2H-1,4-benzoxazine has been performed with catalysts 14 and 16 (Scheme 5). The enantioselectivity was found to be 99% when 14 was used; and the use of the polymeric network 16 afforded a product with an ee of 98%. The result clearly shows that nearly no loss in ee occurred when switched over to heterogeneous catalysis. The heterogeneous catalyst 16 was easily separated by centrifugation and reused for further repeating runs. For each run, 99% conversion and 98% ee were observed and even after 10 runs, the catalyst works without any loss in activity or enantioselectivity (Scheme 5).
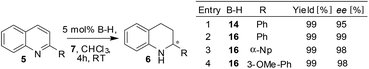
Not only benzoxazines but also quinolines were found to be hydrogenated under the established heterogeneous conditions. For instance, the asymmetric transfer hydrogenation reaction of 2-arylquinolines was carried out with the polymer network 16 (5→6) and the results showed that the performance of the polymer network is comparable to the homogeneous reaction and gives high ee's for different aryl substituents (Scheme 6).
It must be noted that the Rueping/Sugiono catalyst 4 requires 20–24 h time to achieve full conversion with 94% ee for asymmetric hydrogenation of 8 (Scheme 3) and the Thomas/Blechert catalyst 16 catalyzes the same reaction just in 2 h with enhanced enantioselectivity (Scheme 5). The reaction rate with the heterogeneous catalyst 16 was also monitored by kinetic experiments and found to be as fast as with the homogeneous catalyst 14 owing to its microporous nature and high surface area (Schemes 5 and 6). The heterogeneous catalyst 16 can be easily separated by centrifugation and reused for several runs without any loss in activity or selectivity. The authors performed hot extraction experiments to check whether the catalysis is truly heterogeneous in nature or not. After 50% conversion of substrate 8, they filtered out catalyst 16, and even after 24 h they observed no further conversion of substrate 8 to the product confirming that the catalysis is truly heterogeneous.
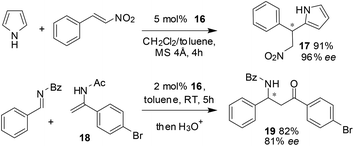
As shown in Scheme 7, the BNPPA-derived catalyst 16 can also be used for the asymmetric Friedel–Crafts alkylation of unprotected pyrroles with nitroalkenes. The reaction in a mixture of DCM and toluene at room temperature afforded a yield of 91% with a selectivity of 96% ee. The heterogeneous catalyst 16 is also very useful in catalyzing an aza-ene-type reaction between (N-(1-(4-bromophenyl)vinyl)acetamide) 18 and (E)-N-benzylidenebenzamide to give the corresponding adduct which on subsequent hydrolysis afforded the β-amino ketone 19 in 82% yield and 81% ee.
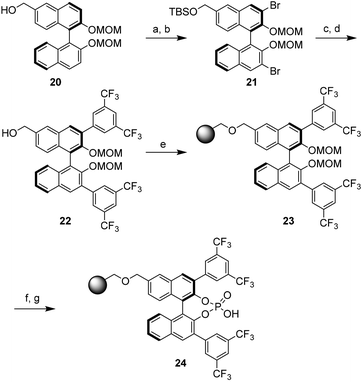
Very recently, Pericàs and coworkers designed polystyrene-supported chiral BINOL derived phosphoric acid.5 As outlined in Scheme 8, it is evident that the immobilization was done at the remote position to avoid perturbation of the active site of the catalyst. The synthesis began with the readily available 6-hydroxymethyl (R)-BINOL derivative 20. Compound 20 was converted to the 6-hydroxymethyl derivative 22 in four steps (cf. 21) and, subsequently, this monomer was anchored onto a Merrifield resin by nucleophilic substitution of chlorine atoms (cf. 23). The desired heterogeneous catalyst 24 was obtained after cleavage of the -MOM group and subsequent phosphoric acid formation followed by HCl wash.6
The immobilized catalyst 24 was found to be highly active for the enantioselective Friedel–Crafts reaction of indoles and sulfonylimine to afford 3-indolylmethanamines in high yields and excellent ee's (Scheme 9). It is interesting to note that the results obtained with this catalyst were comparable to those reported by You and co-workers with a homogeneous Brønsted acid catalyst.7 The report by Pericàs and coworkers clearly demonstrates two striking observations over the superiority of heterogeneous catalysts to homogeneous catalysts: (1) with the PS-supported catalyst 24, good ee's were obtained at room temperature and there is no need of cooling the reaction mixture at –68 °C; (2) the reaction works well only with 1.5 equivalents of indole and a large amount of indole is not necessary.
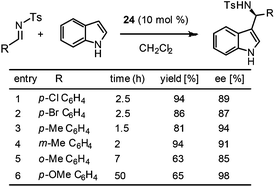 | ||
| Scheme 9 Enantioselective Friedel–Crafts reaction of indoles and sulfonylimine catalysed by the PS-supported chiral Brønsted acid 24. | ||
The catalyst proved to be recyclable up to six cycles without a drop of yield and ee. However, a small drop in ee was observed in the seventh cycle. Very interestingly, the authors found that the activity of the catalyst could be regained with a simple acidic wash (HCl in EtOAc) of the catalyst. Notably, the reactivated catalyst was even more active than the initial one, which allowed for seven more cycles without any significant loss in activity or ee. This observation could be attributed to the presence of phosphate salt that remained during HCl work up in the final stage of phosphoric acid preparation.6 Thus, the authors decided to use HCl/EtOAc washings as the last stage of the preparation of 24.
The research group of Pericàs further endeavoured to use the PS-supported catalyst 24 for designing a single-pass, continuous flow reactor.8 It should be noted that the use of a chiral Brønsted acid in designing a continuous flow micro-reactor was shown for the first time by Rueping et al.9 However, in this case the monomeric catalyst was pumped into the solution and thus the advantages of a supported catalyst could not be fully exploited. With Pericàs's continuous flow reactor, N-Ts imine derived from tosylimine and p-tolualdehyde, reacted with indole to obtain corresponding product in 80% yield and 94% ee.
Conclusions
This highlight describes the design, synthesis and potential applications of heterogeneous Brønsted acid catalysts. The fact that the heterogeneous catalysts are recoverable and can be easily reused several times would surely provide an impetus to the area of enantioselective chiral Brønsted acid catalysis in industries or in large scale synthesis. While the immediate future research is most likely to expand the concept to a broader range of substrates with the design of new polymeric heterogeneous catalysts, it is the author's belief that these reports would give birth to many research areas. A few ideas, though they are highly speculative, can easily be envisioned in the area of merging heterogeneous enantioselective Brønsted acid catalysis with homogeneous/heterogeneous10 (1) organocatalysis, (2) metal catalysis, and (3) enzyme catalysis. In addition, it will be interesting to see whether synergism,11 which is fairly common in homogeneous catalysis, would take place when two catalysts (either homogeneous or heterogeneous) employed are chiral. Similarly, one may ask whether the asymmetric chiral counter anion catalysis (ACDC) strategy12 which is very powerful in the case of chiral phosphoric acid catalysis can be extended to polymeric Brønsted acids. The future efforts would more likely be centered on these areas and we believe the present highlight has set up an appropriate background.Acknowledgements
We gratefully acknowledge financial support by Department of Science and Technology (No. SB/S1/OC-17/2013), India for our research in related area. The generous grants from CSIR, New Delhi (ORGIN program under XII Five Year Plan) and start-up grant from NCL, Pune has also been gratefully acknowledged.References
- For reviews on enantioselective Brønsted acid catalysis, see: (a) J. Yu, F. Shi and L.-Z. Gong, Acc. Chem. Res., 2011, 44, 1156–1171 CrossRef CAS PubMed; (b) M. Terada, Synthesis, 2010, 1929 CrossRef CAS PubMed; (c) A. Zamfir, S. Schenker, M. Freund and S. B. Tsogoeva, Org. Biomol. Chem., 2010, 8, 5262–5276 CAS; (d) T. Akiyama, Chem. Rev., 2007, 107, 5744–5758 CrossRef CAS PubMed; (e) M. Terada, Chem. Commun., 2008, 4097–4112 RSC; (f) M. Terada, Bull. Chem. Soc. Jpn., 2010, 83, 101–119 CrossRef CAS; (g) D. Kampen, C. M. Reisinger and B. List, Top. Curr. Chem., 2009, 291, 395–456 CrossRef.
- D. E. Bergbreiter and S. Kobayashi, Chem. Rev., 2009, 109, 257–838 CrossRef CAS PubMed.
- M. Rueping, E. Sugiono, A. Steck and T. Theissmann, Adv. Synth. Catal., 2010, 352, 281–287 CrossRef CAS PubMed.
- D. S. Kundu, J. Schmidt, C. Bleschke, A. Thomas and S. Blechert, Angew. Chem., Int. Ed., 2012, 51, 5456–5459 CrossRef CAS PubMed . Also see: C. Bleschke, J. Schmidt, D. S. Kundu, S. Blechert and A. Thomas, Adv. Synth. Catal., 2011, 353, 3101–3106 CrossRef PubMed.
- L. Osorio-Planes, C. Rodríguez-Escrich and M. A. Pericàs, Chem. – Eur. J., 2014, 20, 2367–2372 CrossRef CAS PubMed.
- (a) M. Klussmann, L. Ratjen, S. Hoffmann, V. Wakchaure, R. Goddard and B. List, Synlett, 2010, 2189 CrossRef CAS PubMed; (b) M. Hatano, K. Moriyama, T. Maki and K. Ishihara, Angew. Chem., Int. Ed., 2010, 49, 3823–3826 CrossRef CAS PubMed.
- Q. Kang, Z.-A. Zhao and S.-L. You, J. Am. Chem. Soc., 2007, 129, 1484–1485 CrossRef CAS PubMed.
- For reviews on continuous flow processes, see: (a) C. Wiles and P. Watts, Green Chem., 2012, 14, 38–54 RSC; (b) R. M. Myers, K. A. Roper, I. R. Baxendale and S. V. Ley, The Evolution of Immobilized Reagents and their Application in Flow Chemistry for the Synthesis of Natural Products and Pharmaceutical Compounds, in Modern Tools for the Synthesis of Complex Bioactive Molecules, ed. J. Cossy and S. Arseniyadis, John Wiley & Sons, Inc., Hoboken, NJ, USA, 2012 DOI:10.1002/9781118342886.ch11; (c) J. Yoshida, Chem. Rec., 2010, 10, 332–341 CrossRef CAS PubMed; (d) B. P. Mason, K. E. Prince, J. L. Steinbacher, A. R. Bogdan and D. T. McQuade, Chem. Rev., 2007, 107, 2300–2318 CrossRef CAS PubMed; (e) A. Kirschning, W. Solodenko and K. Mennecke, Chem. – Eur. J., 2006, 12, 5972–5990 CrossRef CAS PubMed.
- H.-H. Liao, C.-C. Hsiao, E. Sugiono and M. Rueping, Chem. Commun., 2013, 49, 7953–7955 RSC.
- Reviews: (a) N. T. Patil, V. S. Shinde and B. Gajula, Org. Biomol. Chem., 2012, 10, 211–224 RSC; (b) N. T. Patil, Angew. Chem., Int. Ed., 2011, 50, 6216–6232 CrossRef PubMed; (c) J. Zhou, Chem. – Asian J., 2010, 05, 422–434 CrossRef CAS PubMed; (d) C. Zhong and X. Shi, Eur. J. Org. Chem., 2010, 2999–3025 CrossRef CAS PubMed; (e) Z. Shao and H. Zhang, Chem. Soc. Rev., 2009, 38, 2745–2755 RSC.
- A. E. Allen and D. W. C. MacMillan, Chem. Sci., 2012, 3, 633–658 RSC.
- (a) S. Mayer and B. List, Angew. Chem., Int. Ed., 2006, 45, 4193–4195 CrossRef CAS PubMed; (b) G. L. Hamilton, E. J. Kang, M. Mba and F. D. Toste, Science, 2007, 317, 496–499 CrossRef CAS PubMed.
| This journal is © the Partner Organisations 2014 |