A general method for asymmetric arylation and vinylation of silyl ketene acetals†
Junfeng
Yang
and
Jianrong (Steve)
Zhou
*
Division of Chemistry and Biological Chemistry, School of Physical and Mathematical Sciences, Nanyang Technological University, 21 Nanyang Link, Singapore 637371, Singapore. E-mail: jrzhou@ntu.edu.sg; Fax: (+65)67911961
First published on 18th March 2014
Abstract
A new biarylmonophosphine was developed for highly asymmetric arylation and vinylation of silyl enolates of acyclic esters with good generality. The new stereocenters α to the ester groups were formed in high enantiomeric excess. The method was applied to the asymmetric synthesis of Profen drugs on a gram scale.
In the development of asymmetric α-arylation of carbonyl compounds, the main driving force is the need to prepare enantiopure Profens. Profens are a family of nonsteroidal anti-inflammatory drugs, including over-the-counter painkillers such as Ibuprofen, Naproxen, and Ketoprofen. They all contain the core structure of α-arylpropionic acids having tertiary stereocenters at the α positions.1 Profen enantiomers are known to possess significantly different pharmacological profiles. The (S) isomers are more biologically active than the (R) forms. Consequently, Naproxen is sold solely in (S) form. Today, to access α-arylcarboxylic acids and derivatives, resolution2 and asymmetric C–C couplings3 are common. Among them, direct asymmetric coupling between aryl electrophiles and enolates is one of the most efficient ways to access these compounds. In the past decade, a number of α-arylations of enolates have been developed to form quaternary centers in high ee (Fig. 1a).4 The enolates were generated in situ from strong bases and carbonyl compounds including lactones,5 ketones,6 aldehydes7 and oxindoles.8 The use of strong bases prevented these methods from being used for the construction of tertiary α-stereocenters, due to the facile racemization of those products under basic conditions. Recently, we realized α-arylation of enolates in high ee which produced tertiary α-stereocenters. To prevent product racemization, silicon and tin enolates of esters,9 lactones10 and ketones11 were used (Fig. 1b). Other related metal-catalyzed methods were also reported. Examples include Cu-catalyzed coupling of diaryliodonium salts and soft enolates12 and Ni-catalyzed coupling of α-bromoesters and aryl-metal reagents (Fig. 1c).13
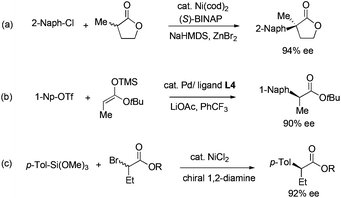 | ||
| Fig. 1 Asymmetric C–C couplings to prepare α-arylesters and α-aryllactones (structure of L4, see Fig. 2). | ||
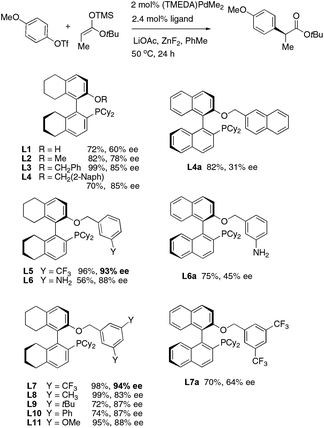
In our previously reported α-arylation of esters using chiral ligand L4, most aryl triflates carrying para-groups gave <90% ee.9 For example, the coupling of p-anisyl triflate and t-butyl propionate resulted in 85% ee and the reaction stopped after partial conversion.
We decided to use the model coupling between p-anisyl triflate and a trimethylsilyl enolate derived from t-butyl propionate to seek a more stereoselective catalyst (Fig. 2). Based on our past experience in arylation of ketones11 and lactones,10 we hypothesized that the modification of the O-benzyl side arm of ligand L4 may help. Indeed monophosphines L5 and L7 carrying m-CF3-benzyl groups led to 93% and 94% ee, respectively. Most other modifications on the benzyl group led to inferior selectivity. In comparison, similar biarylphosphines on a 1,1′-binaphthyl backbone afforded only 30–64% ee.
The choice of other reaction parameters was also crucial to good ee. (TMEDA)PdMe2 was the optimal Pd source and Pd(OAc)2 also gave good yields. LiOAc was essential to facilitate efficient enolate transfer. The ZnF2 additive (0.2 equiv.) can further accelerate the process. In terms of choice of solvents, good yields can also be obtained in toluene, benzene, o-xylene and diethyl ether. In PhCF3, the model reaction of anisyl triflate was much slower and stopped after partial conversion.
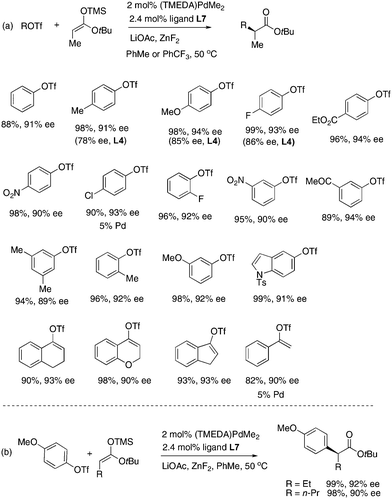
The Pd/L7 catalyst was successfully applied to many structurally diverse aryl triflates (Fig. 3a). In almost all of cases ligand L7 was more stereoselective than ligand L4.9 Both electron-donating and electron-withdrawing groups can be present on the aryl rings. For an electron-neutral or electron-rich ArOTf, better ee was obtained in toluene than in PhCF3. For an electron-poor ArOTf, however PhCF3 was a better solvent. Notably, several alkenyl triflates also coupled well in toluene solvent. Silyl ketene acetals of n-butylate and valerate also coupled well (Fig. 3b).
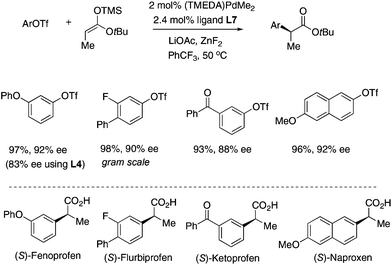
The Pd/L7 catalyst was successfully applied to the asymmetric synthesis of some Profens including Fenoprofen, Flurbiprofen, Ketoprofen and Naproxen (Fig. 4). In most cases, the coupling proceeded smoothly in >90% ee.14 The t-butyl esters of the products can be easily hydrolyzed to release the Profens using trifluoroacetic acid. After one crystallization the ee of synthetic Flurbiprofen was improved to 96% (84% yield), and after recrystallization to 99%. The absolute configuration of synthetic Naproxen was determined to be (2S) by comparison with the reported optical rotation.15
In summary, we report herein a general Pd catalyst for asymmetric arylation and vinylation of ester enolates that formed tertiary carbon centers. The enantioselectivity was uniformly high as compared to our previous report in 2011.9 The method allows a quick access to many Profen analogues in >90% ee with a general scope. In our recent asymmetric arylations of cyclic ketones and lactones, weak CH⋯O hydrogen bonding was found to be responsible for asymmetric induction and the C–C reductive elimination was the stereo-determining step.10,11 In the arylation of silyl enolates of acyclic esters, however it is probably transmetalation that dictates the stereochemical outcome, since (E) and (Z) isomers of a silyl ketene acetal gave significantly different ee values during arylation.9
We thank the Singapore National Research Foundation (NRF-RF2008-10) and Nanyang Technological University for financial support. We thank Johnson Matthey for a gift of palladium salts.
Notes and references
- (a) M. F. Landoni and A. Soraci, Curr. Drug Metab., 2001, 2, 37 CrossRef CAS; (b) P. J. Harrington and E. Lodewijk, Org. Process Res. Dev., 1997, 1, 72 CrossRef CAS.
- (a) Kinetic resolution via esterification: I. Shiina, K. Nakata, K. Ono, Y.-s. Onda and M. Itagaki, J. Am. Chem. Soc., 2010, 132, 11629 CrossRef CAS PubMed; (b) Selective crystallization using a chiral amine: Y. Chikusa, T. Fujimoto, M. Ikunaka, T. Inoue, S. Kamiyama, K. Maruo, J. Matsumoto, K. Matsuyama, M. Moriwaki, H. Nohira, S. Saijo, M. Yamanishi and K. Yoshida, Org. Process Res. Dev., 2002, 6, 291 CrossRef CAS; (c) Resolution of esters using hydrolases: Y. Chikusa, Y. Hirayama, M. Ikunaka, T. Inoue, S. Kamiyama, M. Moriwaki, Y. Nishimoto, F. Nomoto, K. Ogawa, T. Ohno, K. Otsuka, A. K. Sakota, N. Shirasaka, A. Uzura and K. Uzura, Org. Process Res. Dev., 2003, 7, 289 CrossRef CAS; (d) Resolution using lipases: Q. Wu, P. Soni and M. T. Reetz, J. Am. Chem. Soc., 2013, 135, 1872 CrossRef CAS PubMed; (e) Resolution of azolides by lipases: P.-Y. Wang, Y.-J. Chen, A.-C. Wu, Y.-S. Lin, M.-F. Kao, J.-R. Chen, J.-F. Ciou and S.-W. Tsai, Adv. Synth. Catal., 2009, 351, 2333 CrossRef CAS.
- (a) Asymmetric alkylation using chiral amide enolates: A. G. Myers, B. H. Yang, H. Chen, L. McKinstry, D. J. Kopecky and J. L. Gleason, J. Am. Chem. Soc., 1997, 119, 6496 CrossRef CAS; (b) Alkylation using chiral lithium amides: C. E. Stivala and A. Zakarian, J. Am. Chem. Soc., 2011, 133, 11936 CrossRef CAS PubMed; (c) Asymmetric hydroformylation: J. Klosin and C. R. Landis, Acc. Chem. Res., 2007, 40, 1251 CrossRef CAS PubMed; (d) Asymmetric hydroformylation: K. Nozaki, N. Sakai, T. Nanno, T. Higashijima, S. Mano, T. Horiuchi and H. Takaya, J. Am. Chem. Soc., 1997, 119, 4413 CrossRef CAS; (e) Asymmetric hydrovinylation: G. Franciò, F. Faraone and W. Leitner, J. Am. Chem. Soc., 2002, 124, 736 CrossRef PubMed; (f) Asymmetric hydrovinylation: C. R. Smith and T. V. RajanBabu, Org. Lett., 2008, 10, 1657 CrossRef CAS PubMed.
- (a) Reviews: F. Bellina and R. Rossi, Chem. Rev., 2010, 110, 1082 CrossRef CAS PubMed; (b) C. C. C. Johansson and T. J. Colacot, Angew. Chem., Int. Ed., 2010, 49, 676 CrossRef CAS PubMed; (c) A. C. B. Burtoloso, Synlett, 2009, 320 CrossRef CAS PubMed.
- Asymmetric arylation of lactones: D. J. Spielvogel and S. L. Buchwald, J. Am. Chem. Soc., 2002, 124, 3500 CrossRef CAS PubMed.
- (a) Asymmetric arylation of ketone enolates: J. Åhman, J. P. Wolfe, M. V. Troutman, M. Palucki and S. L. Buchwald, J. Am. Chem. Soc., 1998, 120, 1918 CrossRef; (b) X. Liao, Z. Weng and J. F. Hartwig, J. Am. Chem. Soc., 2008, 130, 195 CrossRef CAS PubMed; (c) T. Hamada, A. Chieffi, J. Åhman and S. L. Buchwald, J. Am. Chem. Soc., 2002, 124, 1261 CrossRef CAS PubMed; (d) G. Chen, F. Y. Kwong, H. O. Chan, W.-Y. Yu and A. S. C. Chan, Chem. Commun., 2006, 1413 RSC; (e) S. Ge and J. F. Hartwig, J. Am. Chem. Soc., 2011, 133, 16330 CrossRef CAS PubMed.
- (a) J. García-Fortanet and S. L. Buchwald, Angew. Chem., Int. Ed., 2008, 47, 8108 CrossRef PubMed; (b) P. Nareddy, L. Mantilli, L. Guénée and C. Mazet, Angew. Chem., Int. Ed., 2012, 51, 3826 CrossRef CAS PubMed.
- (a) Asymmetric arylation of oxindoles: A. M. Taylor, R. A. Altman and S. L. Buchwald, J. Am. Chem. Soc., 2009, 131, 9900 CrossRef CAS PubMed; (b) S. Lee and J. F. Hartwig, J. Org. Chem., 2001, 66, 3402 CrossRef CAS PubMed.
- Z. Huang, Z. Liu and J. Zhou, J. Am. Chem. Soc., 2011, 133, 15882 CrossRef CAS PubMed.
- Z. Huang, Z. Chen, L. H. Lim, G. C. P. Quang, H. Hirao and J. Zhou, Angew. Chem., Int. Ed., 2013, 52, 5807 CrossRef CAS PubMed.
- Z. Huang, L. H. Lim, Z. Chen, Y. Li, F. Zhou, H. Su and J. Zhou, Angew. Chem., Int. Ed., 2013, 52, 4906 CrossRef CAS PubMed.
- (a) A. E. Allen and D. W. C. MacMillan, J. Am. Chem. Soc., 2011, 133, 4260 CrossRef CAS PubMed; (b) J. S. Harvey, S. P. Simonovich, C. R. Jamison and D. W. C. MacMillan, J. Am. Chem. Soc., 2011, 133, 13782 CrossRef CAS PubMed; (c) A. Bigot, A. E. Williamson and M. J. Gaunt, J. Am. Chem. Soc., 2011, 133, 13778 CrossRef CAS PubMed; (d) E. Skucas and D. W. C. MacMillan, J. Am. Chem. Soc., 2012, 134, 9090 CrossRef CAS PubMed.
- X. Dai, N. A. Strotman and G. C. Fu, J. Am. Chem. Soc., 2008, 130, 3302 CrossRef CAS PubMed.
- (a) A recent example of asymmetric arylation of ester for Flurbiprofen synthesis in 90% ee using TlOAc and Pd/Josiphos catalyst: H. Yoshida, R. Yoshida and K. Takaki, Angew. Chem., Int. Ed., 2013, 52, 8629 CrossRef CAS PubMed; (b) K. Kobayashi, Y. Yamamoto and N. Miyaura, Organometallics, 2011, 30, 6323 CrossRef CAS.
- I. T. Harrison, B. Lewis, P. Nelson, W. Rooks, A. Roszkowski, A. Tomolonis and J. H. Fried, J. Med. Chem., 1970, 13, 203 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures for asymmetric coupling and characterization of new compounds. See DOI: 10.1039/c4qo00027g |
| This journal is © the Partner Organisations 2014 |