Naphthodithieno[3,2-b]thiophene-based semiconductors: synthesis, characterization, and device performance of field-effect transistors†
Ji
Zhang‡
a,
Zhaoguang
Li‡
b,
Hui
Xing
b,
Weifeng
Zhang
a,
Lei
Guo
b,
Yunqi
Liu
a,
Man Shing
Wong
*b and
Gui
Yu
*a
aBeijing National Laboratory for Molecular Sciences, Institute of Chemistry, Chinese Academy of Sciences, Beijing 100190, P. R. China. E-mail: yugui@iccas.ac.cn
bInstitute of Molecular Functional Materials, Department of Chemistry, and Institute of Advanced Materials, Hong Kong Baptist University, Kowloon Tong, Hong Kong SAR, P. R. China. E-mail: mswong@hkbu.edu.hk
First published on 21st March 2014
Abstract
A series of novel naphthodithieno[3,2-b]thiophene (NDTT) derivatives were successfully synthesized. Organic field-effect transistors based on these compounds were fabricated and the device performances were investigated in detail. By means of simple thermal annealing, the mobilities of NDTT-10 and NDTT-12 are dramatically improved from the order of 10−3 up to 0.22 and 0.13 cm2 V−1 s−1, respectively. Furthermore, the structure–functional property relationships of these compounds are also discussed. NDTT is the new building block indeed, and further modification of the NDTT π-backbone would afford diverse new small molecular and polymeric π-functional materials for organic electronics.
Organic π-conjugated semiconductors have attracted great attention since the 1980s for the development of next-generation electronics,1–4 for instance organic light emitting diodes, organic field-effect transistors (OFETs)5,6 and organic photovoltaic devices (OPVs).7 A large number of organic semiconductors for OFETs have been synthesized and investigated, including various small molecules4 and polymers.6,8–11 As the representative semiconductors, pentacene,12,13 oligo/polythiophenes,1,13 and C60 (ref. 14) have been widely investigated in theoretical and experimental studies in the last two decades. Thienoacenes are a class of fused aromatic molecules composed of thiophenes and benzene rings.11 Owing to the possible existence of intermolecular CH⋯π, π⋯π and/or S⋯S interactions in the solid state of thienoacenes, holes and electrons could be easily transported between adjacent molecules.15,16 To date, several thienoacene derivatives have been proved to show high mobilities exceeding 0.1 cm2 V−1 s−1 including 2,7-diphenyl[1]benzothieno[3,2-b]-benzothiophene,17,18 dinaphtho-[2,3-b:2′,3′-f]thieno[3,2-b]thiophene,19 and dianthra[2,3-b:2′,3′-f]-thieno[3,2-b]thiophene.20 Their solubility and mobility could further be efficiently modulated by varying the alkyl side-groups, and good solubility was considered as a key factor for a low-cost technology. In general, appropriate long and branched alkyl groups often give rise to a close molecular packing due to the better van der Waals intermolecular interactions, arising from the nature of self-organization, which is a useful tool and approach to enhance carrier transport.18 The air instability of the OFET devices is another problem that hinders the practical applications of most organic materials. Pentacene, oligo-/polythiophenes, and diketopyrrolopyrrole-containing conjugated polymers suffer from oxidative instability.21 Nevertheless, the introduction of fused thiophene rings to lower the energy levels of highest occupied molecular orbitals (HOMOs) could alleviate this problem.11 In general, air-stable p-channel organic transistors can be realized when the HOMO energy level of the material is lower than −5.0 eV.22 Compared to typical thienoacenes, such as [n]thienoacenes ([n]TACs) and benzene–thiophene alternating molecules (BTAs), naphthodithiophenes with the naphthalene group tend to exhibit a more extended π-conjugated delocalization and hence smaller energy gap, which often benefits the performance of OFETs and OPVs.19,23–25 Thus, naphthodithiophenes could be potential candidates for future applications. Recently, a few organic semiconductors based on naphthodithiophene were synthesized and shown to exhibit high performance in OFETs and OPVs.21,26–29 In the present work, a novel series of air-stable and soluble naphthodithieno[3,2-b]thiophene (NDTT) derivatives with different side-alkyl groups were designed and synthesized, and the OFETs based on these materials were also fabricated and investigated.
Results and discussion
Synthesis
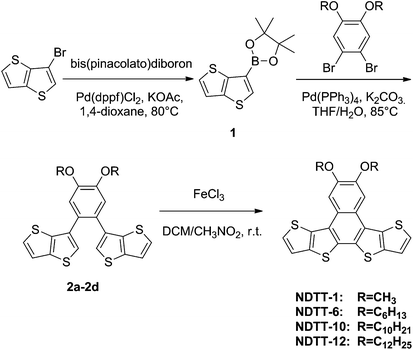
Synthesis of four naphthodithienothiophene derivatives, NDTT-n (n = 1, 6, 10, and 12), is shown in Scheme 1 which was briefly described as follows. 2-(4,4,5,5-Tetramethyl)-1,3,2-dioxaboroan-2-yl-thieno[3,2-b]-thiophene, 1, was synthesized in 60% yield from 3-bromothieno-thiophene and bis(pinacolato)-diboron according to the literature procedures.30,31 Various 1,2-dibromo-4,5-dialkoxylbenzenes were synthesized according to the literature procedure.29 The palladium-catalysed Suzuki coupling of thienothiophene–boronic acid derivative 1 with 1,2-dibromo-4,5-dialkoxyl-benzenes gave the desired intermediates 2a–d in moderate yields of 50–70%. Subsequent FeCl3-catalyzed oxidative cyclization afforded the target naphthodithieno-thiophenes, NDTT-n, in 40–50% yield. All the new naphthodithienothiophenes were fully characterized by 1H NMR and 13C NMR spectroscopy, elemental analysis, cyclic voltammetry, and mass spectrometry (see Experimental procedures, ESI†). In addition, thermogravimetric analysis (TGA) was carried out for all NDTT-n (n = 1, 6, 10, and 12) which indicates little weight loss before 350 °C, suggesting good thermostabilities of these materials (Fig. S1, ESI†).Optical and electrochemical characterization
Fig. 1a shows the UV–vis absorption spectra of NDTT-n (n = 1, 6, 10, and 12) in CH2Cl2 solution. All the molecules show almost identical absorption peaks at 244, 279, 353, and 372 nm, suggesting the relatively small absorption changes with different alkyl groups. The optical energy gap of these naphthodithienothiophenes is estimated to be 3.25 eV as determined by extrapolating the long-wavelength absorption edge.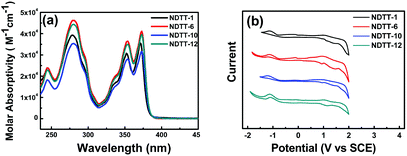 | ||
| Fig. 1 (a) UV–vis absorption spectra measured in CH2Cl2 solution and (b) cyclic voltammograms of NDTT-n (n = 1, 6, 10, and 12). | ||
The onset oxidation (Eonsetoxd) and onset reduction potential (Eonsetred) were estimated by the cyclic voltammetry (CV) method in CH2Cl2 using a platinum disc electrode as a working electrode, platinum wire as a counter electrode, and a saturated calomel electrode as the reference electrode using 0.1 M of Bu4NPF6 as a supporting electrolyte at a scan rate of 100 mV s−1. All the potentials were calibrated with ferrocene as an external standard. The results of the CV measurements of NDTT-n series are summarized in Fig. 1b and Table 1. The HOMO and LUMO energy levels estimated from the CV data are tabulated in Table 1. It is worth mentioning that the HOMO energy levels of these naphthodithienothiophenes are generally lower than that of pentacene (−5.0 eV), implying that they would be more air-stable in energy than pentacene. In addition, the band gaps of NDTT-n estimated from CV results are in the range of 2.08–2.27 eV, which are much different from the optical band gap of 3.25 eV. The deviation of about 1.0 eV is due to the different aggregation state in solution compared to the solid state in film.
Device properties
OFET devices were fabricated in a bottom-gate bottom-contact (BGBC) configuration (gold electrode on Si/SiO2 substrates). Before the deposition of organic semiconductors, octadecyltrichlorosilane (OTS) treatment was performed on the gate dielectrics which were placed in a vacuum oven with OTS at a temperature of 120 °C for 3 h to form an OTS self-assembled monolayer. Then the NDTT-n thin films were deposited on the substrates by vacuum evaporation. The transistors based on these compounds exhibit typical p-type OFET characteristics. The OFET characteristics of the devices are summarized in Table 2. Typical transfer and output characteristics of the OFET devices based on NDTT-10 and NDTT-12 are shown in Fig. 2. The transfer curve contains the y-axis of the square root of the current, which is theoretically in a linear relation with the gate voltage in the saturated region. The output figure contains several curves under different gate voltages, implying the linear region and saturated region in electrical measurements.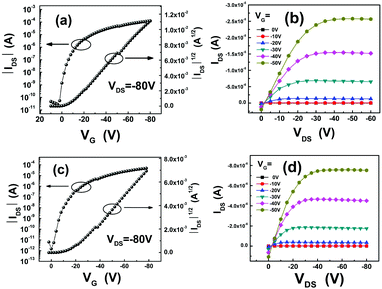 | ||
| Fig. 2 Typical electrical characteristics of the devices: (a) transfer and (b) output characteristics of NDTT-10 based OFETs; (c) transfer and (d) output characteristics of the NDTT-12 based OFETs. | ||
| Compda | μ[cm2 V−1 s−1] | I on/Ioff | T annealing [°C] |
|---|---|---|---|
| a Vacuum evaporation: the organic thin films of all compounds were deposited 10 nm at a rate of 5–10 Å min−1, and then deposited 25 nm at the rate of 15–20 Å min−1. | |||
| NDTT-1 | 5 × 10−4 | 102–103 | RT |
| NDTT-6 | 0.08 | 106–107 | 80 |
| NDTT-10 | 0.22 | 107 | 80 |
| NDTT-12 | 0.13 | 107–108 | 80 |
In order to get the best crystallinity for high performance, the OFET devices fabricated by vacuum evaporation were annealed successively from 60 to 160 °C by the step of 10 °C for 5 min in air, and measured after each annealing step at room temperature in air. In contrast, the characteristics of as-prepared devices were also measured. The mobilities of devices annealed at different temperatures are shown in Fig. 3. At an annealing temperature of 80 °C, the compounds NDTT-10 and NDTT-12 show the highest mobilities of 0.22 and 0.13 cm2 V−1 s−1, respectively. Compared with the relatively low hole mobilities on the order of 10−3 cm2 V−1 s−1 for both as-prepared devices, this remarkable mobility enhancement is uncommon for vacuum-evaporated devices of small molecules.
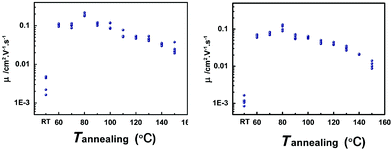 | ||
| Fig. 3 Mobilities of OFETs based on (a) NDTT-10 and (b) NDTT-12 thin films at different annealing temperatures. | ||
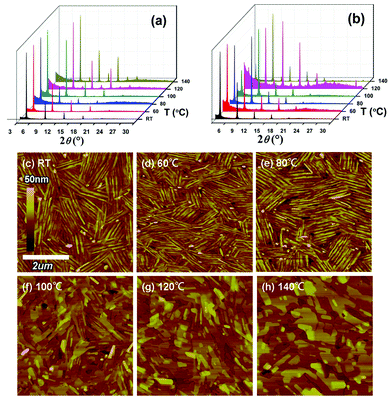
XRD and AFM characterization
To understand the relationships between film crystallinity/morphology and device performance, XRD and AFM investigations of the NDTT-10 and NDTT-12 thin films were carried out and the resulting XRD patterns and AFM images are shown in Fig. 4, Fig. S2 and Table S1.† XRD measurements of the NDTT-10 thin films annealed at temperatures from rt to 100 °C exhibit diffraction peaks at 4.40–4.46, 8.72–8.80, 13.04–13.12, 17.36–17.48, and 21.76–21.92°, which correspond to the first, second, third, fourth, and fifth-order diffraction peaks, respectively, and the corresponding d-spacing was determined to be ∼1.99 nm (Fig. 4a). With the increase of annealing temperature to higher than 120 °C, the primary set of peaks sharply decrease while a new set of peaks appears at 5.44–5.48 and 10.78–10.86°, indicating a new phase formed with a d-spacing of about 1.62 nm. Compared to the well-organized lattice formed at an annealing temperature lower than 100 °C with a d-spacing of 1.99 nm, the shortened d-spacing leads to a close molecular packing, in which the molecules tend to incline at a small angle to the substrate. This is considered to be an adverse effect for the two-dimensional charge transport, which is consistent with the experimental data. Moreover, the polymorphism is composed of more than two types of crystal lattices in the solid state resulting in the formation of carrier barriers at the interface of different forms, and thus lowering the charge mobility. For NDTT-12, the XRD measurements show a similar trend. At all annealing temperatures, NDTT-12 exhibits a set of peaks at 4.00–4.04, 7.86–7.94, 11.78–11.86, 15.66–15.78, and 19.54–19.72°, corresponding to the first, second, third, fourth, and fifth-order diffraction, respectively, which translates into the d-spacing of 2.20 nm. Due to the longer alkyl chains, the larger d-spacing of NDTT-12 than that of NDTT-10 is easy to be explained.18 With the increase of annealing temperature to higher than 120 °C, several new peaks appeared at 9.80–9.84, 13.66–13.72, 17.54–17.64, 21.34–21.56, and 28.44°, suggesting the formation of polymorphs, which accounts for the decrease of charge mobility.As shown in AFM images, both the NDTT-10 and NDTT-12 thin films exhibit a similar morphological change upon annealing under the same conditions (Fig. 4c–h and Fig. S2, ESI†). With the increase of annealing temperature above 100 °C, the grain style gradually changes from a club-like to a tile-like shape, which corresponds to the formation of a new phase as illustrated in XRD studies. It is worth noting that high crystallinity could be found in both the XRD patterns and AFM images for both molecules at RT. Although the NDTT-10 thin film is highly crystalline, large numbers of grain boundaries resulting from the rapid deposition process could explain the relatively low mobility for as-prepared devices at RT. However, this could be filled with the adjacent molecules induced by a higher temperature annealing. In fact, other small molecules such as n-type N,N-ditridecyl-3,4,9,10-perylenetetra-carboxylic diimide (PTCDI-C13)32 also show a similar morphological change, in which the grain style changes from a ball-like to a tile-like shape in the thin film when annealed at 140 °C, while several peaks appeared in XRD patterns.
Conclusions
In summary, we developed four highly π-extended NDTT-based derivatives. NDTT is the brand-new building block and could be further modified to afford functional materials for organic electronics. The OFET devices based on NDTT-10 and NDTT-12 exhibit a promising mobility up to 0.22 and 0.13 cm2 V−1 s−1, respectively, with a current on/off ratio for both exceeding 107 under an ambient atmosphere. These findings highlight the potential of naphthodithienothiophene-containing semiconducting molecules to achieve high-performance OFETs. Meanwhile, a simple thermal annealing technique is used to enhance the vapor-evaporated OFET performance, which is proved to be an effective method.Acknowledgements
This research was financially supported by the National Science Foundation of China (21021091 and 51233006), the Major State Basic Research Development Program (2011CB808403), Guangdong Provincial Science and Technology Project (2012B050300012), and the Chinese Academy of Sciences. This work was also supported by GRF (HKBU 203212), Hong Kong Research Grant Council, the Strategic Development Fund (SDF13-0531-A02) of Hong Kong Baptist University and the Institute of Molecular Functional Materials which was supported by a grant from the University Grants Committee, Areas of Excellence Scheme (AoE/P-03/08).Notes and references
- A. Tsumura, H. Koezuka and T. Ando, Appl. Phys. Lett., 1986, 49, 1210 CrossRef CAS.
- M. L. Zhu, H. Luo, L. P. Wang, G. Yu and Y. Q. Liu, Acta Chim. Sin., 2012, 70, 1599 CrossRef CAS.
- C. D. Dimitrakopoulos and P. R. Malenfant, Adv. Mater., 2002, 14, 99 CrossRef CAS.
- A. Pron, P. Gawrys, M. Zagorska, D. Djurado and R. Demadrille, Chem. Soc. Rev., 2010, 39, 2577 RSC.
- M. Muccini, Nat. Mater., 2006, 5, 605 CrossRef CAS PubMed.
- C. L. Wang, H. L. Dong, W. P. Hu, Y. Q. Liu and D. B. Zhu, Chem. Rev., 2012, 112, 2208 CrossRef CAS PubMed.
- S. Beaupré, P. L. T. Boudreault and M. Leclerc, Adv. Mater., 2010, 22, E6 CrossRef PubMed.
- J. E. Anthony, Chem. Rev., 2006, 106, 5028 CrossRef CAS PubMed.
- J. E. Anthony, A. Facchetti, M. Heeney, S. R. Marder and X. W. Zhan, Adv. Mater., 2010, 22, 3876 CrossRef CAS PubMed.
- W. P. Wu, Y. Q. Liu and D. B. Zhu, Chem. Soc. Rev., 2010, 39, 1489 RSC.
- K. Takimiya, S. Shinamura, I. Osaka and E. Miyazaki, Adv. Mater., 2011, 23, 4347 CrossRef CAS PubMed.
- H. Klauk, M. Halik, U. Zschieschang, G. Schmid, W. Radlik and W. Weber, J. Appl. Phys., 2002, 92, 5259 CrossRef CAS.
- Z. Bao, A. Dodabalapur and A. J. Lovinger, Appl. Phys. Lett., 1996, 69, 4108 CrossRef CAS.
- T. D. Anthopoulos, B. Singh, N. Marjanovic, N. S. Sariciftci, A. M. Ramil, H. Sitter, M. Colle and D. M. de Leeuw, Appl. Phys. Lett., 2006, 89, 3 CrossRef.
- J. L. Bredas, D. Beljonne, V. Coropceanu and J. Cornil, Chem. Rev., 2004, 104, 4971 CrossRef CAS PubMed.
- V. Coropceanu, J. Cornil, D. A. da Silva, Y. Olivier, R. Silbey and J. L. Bredas, Chem. Rev., 2007, 107, 926 CrossRef CAS PubMed.
- H. Minemawari, T. Yamada, H. Matsui, J. Tsutsumi, S. Haas, R. Chiba, R. Kumai and T. Hasegawa, Nature, 2011, 475, 364 CrossRef CAS PubMed.
- H. Ebata, T. Izawa, E. Miyazaki, K. Takimiya, M. Ikeda, H. Kuwabara and T. Yui, J. Am. Chem. Soc., 2007, 129, 15732 CrossRef CAS PubMed.
- T. Yamamoto and K. Takimiya, J. Am. Chem. Soc., 2007, 129, 2224 CrossRef CAS PubMed.
- K. Niimi, S. Shinamura, I. Osaka, E. Miyazaki and K. Takimiya, J. Am. Chem. Soc., 2011, 133, 8732 CrossRef CAS PubMed.
- H. Bronstein, Z. Chen, R. S. Ashraf, W. Zhang, J. Du, J. R. Durrant, P. Shakya Tuladhar, K. Song, S. E. Watkins and Y. Geerts, J. Am. Chem. Soc., 2011, 133, 3272 CrossRef CAS PubMed.
- H. Meng, Z. Bao, A. J. Lovinger, B.-C. Wang and A. M. Mujsce, J. Am. Chem. Soc., 2001, 123, 9214 CrossRef CAS PubMed.
- S. W. Shi, X. D. Xie, P. Jiang, S. Chen, L. W. Wang, M. Wang, H. Q. Wang, X. Y. Li, G. Yu and Y. F. Li, Macromolecule, 2013, 46, 3358 CrossRef CAS.
- I. Osaka, T. Abe, S. Shinamura, E. Miyazaki and K. Takimiya, J. Am. Chem. Soc., 2010, 132, 5000 CrossRef CAS PubMed.
- Y. A. Duan, Y. Geng, H. B. Li, J. L. Jin, Y. Wu and Z. M. Su, J. Comput. Chem., 2013, 34, 1611 CrossRef CAS PubMed.
- I. Osaka, T. Kakara, N. Takemura, T. Koganezawa and K. Takimiya, J. Am. Chem. Soc., 2013, 135, 8834 CrossRef CAS PubMed.
- S. R. Sanjaykumar, S. Badgujar, C. E. Song, W. S. Shin, S. J. Moon, I. N. Kang, J. Lee, S. Cho, S. K. Lee and J. C. Lee, Macromolecules, 2012, 45, 6938 CrossRef.
- M. C. Hwang, J. W. Jang, T. K. An, C. E. Park, Y. H. Kim and S. K. Kwon, Macromolecules, 2012, 45, 4520 CrossRef CAS.
- B. Wang, S. W. Tsang, W. F. Zhang, Y. Tao and M. S. Wong, Chem. Commun., 2011, 47, 9471 RSC.
- L. S. Fuller, B. Iddon and K. A. Smith, J. Chem. Soc., Perkin Trans. 1, 1997, 3465 RSC.
- A. Bugge, Acta Chem., Scand., 1969, 23, 2704 CrossRef CAS.
- S. Tatemichi, M. Ichikawa, T. Koyama and Y. Taniguchi, Appl. Phys. Lett., 2006, 89, 112108 CrossRef.
Footnotes |
| † Electronic supplementary information (ESI) available: Experimental procedures and full spectroscopic data for all new compounds. See DOI: 10.1039/c4qo00021h |
| ‡ These authors contributed equally to this work. |
| This journal is © the Partner Organisations 2014 |

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif)