Highly efficient and practical resolution of 2,3:6,7-dibenzobicyclo[3.3.1]nona-2,6-diene-4,8-dione and stereoselective synthesis of its chiral diamine derivatives†
Bin
Wang
and
Zhenhua
Gu
*
Department of Chemistry, University of Science and Technology of China, 96 Jinzhai Road, Hefei, Anhui 230026, P. R. China. E-mail: zhgu@ustc.edu.cn
First published on 26th February 2014
Abstract
Highly efficient and practical resolution of 2,3:6,7-dibenzobicyclo[3.3.1]nona-2,6-diene-4,8-dione by inclusion complexation with commercially available enantiopure 1,1′-bi-2-naphthol is reported. The structure of the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 inclusion complex of the diketone and BINOL was confirmed by single crystal X-ray crystallography.
1 inclusion complex of the diketone and BINOL was confirmed by single crystal X-ray crystallography.
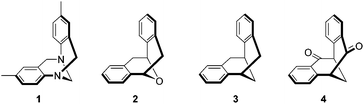
Rigid cleft-like molecules have attracted considerable attention in the area of molecular recognition. One of the most prominent molecules is Tröger’s base 1 (Fig. 1), which was first synthesized more than one hundred and twenty years ago.1 The molecule has a dihedral angle of around 90° and the two phenyl rings are fused to the bicyclic [3.3.1] framework to form a rigid V-shaped scaffold.2 Tröger’s base contains two chiral centers at the nitrogens and it could be resolved by an optically active chiral acid.3 However, in most of the host–guest studies with Tröger’s base,4 enantiopure Tröger’s base has rarely been used to discriminate chiral substrates.5 One of the reasons is that Tröger’s base undergoes partial racemization under acidic conditions via ring-opening and ring-closing processes.6,7 Studies were also carried out on a series of related systems, including Kagan's ether 2,8 dibenzobicyclo[3.3.1]nona-2,6-diene 3,9 and 2,3:6,7-dibenzobicyclo[3.3.1]nona-2,6-diene-4,8-dione 4 (Fig. 1).10 We are interested in this class of cleft-like molecules not only because of their utility in molecular recognition and self-assembly studies, but their potential applications in organic synthesis.11
Our initial studies were focused on diketone 4 as the target molecule, which has the following two merits: (1) in comparison to Tröger’s base, the chirality of 4 is stable under either acidic or basic conditions; and (2) the carbonyl group in 4 provides opportunities for further functionalization of this V-shaped molecule. However, it has been difficult to obtain gram quantities of enantiopure 4 by the known procedures. For example, multiple recrystallizations of isomeric mixtures of diacids (±)-5 and meso-5 were required to isolate pure (±)-5 in a previous synthesis (Scheme 1).10a,12 Resolution of (±)-5 with 1.0 equiv. of quinine afforded only moderate levels of enantioselectivity. Furthermore, the solid salt (+)-5·quinine was found to be thermally unstable under the conditions required for fractional recrystallization.10a,12 Double Friedel–Craft acylation of (+)-5 or (−)-5 provided 4 in good yield, for which the optical purity could be improved further by recrystallization.
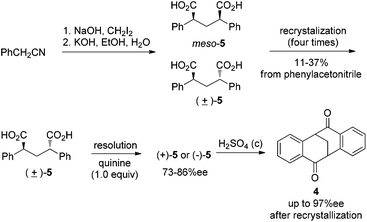 | ||
| Scheme 1 Previous synthesis of enantiopure 4.10a,12 | ||
The direct resolution of (±)-4 has advantages over the previous procedures for resolving diacid since the need for fractional recrystallization of 4 would be entirely bypassed. Ideally, 4 could be synthesized on multi-gram scale from 2-phenylacetonitrile in three steps without chromatography or recrystallization (see ESI† for details).
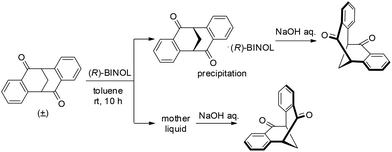
The idea for direct resolution of the diketone by 1,1′-bi-2-naphthol (BINOL) came from an unexpected observation. Mixing a solution of (±)-4 in toluene and a solution of (R)-BINOL in toluene resulted in the immediate formation of a white precipitate.13,14 Thus, after stirring a mixture of (±)-4 and 0.60 equiv. of (R)-BINOL in toluene at rt for 5 min, a solid was collected by filtration and (S)-4 was obtained in 91% ee by decomposition of the complex with aqueous NaOH (Table 1, entry 1). Both the yield and enantioselectivity were increased with a prolonged stirring time (entries 2 and 3). Various ratios of (±)-4 and (R)-BINOL were investigated and it was found that the efficiency of the resolution gradually decreased when the loading of (R)-BINOL was increased (entries 4 and 5). Varying the concentration did not provide better results (entries 6 and 7).
| Entry | Conc. (mol L−1) | Ratio 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) BINOL BINOL |
Precipitation (S)-4 | Mother liquor (R)-4 | ||
|---|---|---|---|---|---|---|
| Yieldb/% | ee% | Yieldb/% | ee% | |||
| a The reactions were conducted at 1.0 mmol scale of (±)-4. b Yields refer to the isolated yields after decomposition with aqueous NaOH. c The mixture was stirred at rt for 5 min. d The mixture was stirred at rt for 1 h. e The reaction was carried out on 12.40 g (50.0 mmol) scale. The results in parentheses are after one recrystallization, for details see ESI. f The ee% was not determined. | ||||||
| 1c | 0.20 | 1.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.60 0.60 |
36 | 91 | 63 | —f |
| 2d | 0.20 | 1.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.60 0.60 |
44 | 93 | 55 | —f |
| 3 | 0.20 | 1.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.60 0.60 |
47 | 95 | 51 | 85 |
| 4 | 0.20 | 1.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.80 0.80 |
47 | 83 | 45 | 84 |
| 5 | 0.20 | 1.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.0 1.0 |
43 | 80 | 48 | 80 |
| 6 | 0.10 | 1.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.60 0.60 |
44 | 92 | 55 | 79 |
| 7 | 0.30 | 1.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.60 0.60 |
53 | 92 | 46 | 68 |
| 8e | 0.20 | 1.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.60 0.60 |
(42) | (>99) | (44) | (>99) |
It is notable that the resolution performs very well for a multi-gram scale preparation of enantioenriched 4. For example, stirring a mixture of (±)-4 (12.40 g, 50.0 mmol) and (R)-BINOL (30.0 mmol) at room temperature for 10 h, followed by filtration and decomposition with aqueous NaOH, provides (S)-4 and (R)-4 in good yields and excellent enantiopurity after one recrystallization (Table 1, entry 8).
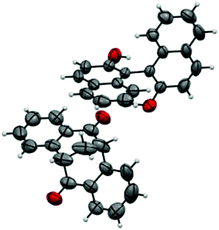
A single crystal of the complex suitable for X-ray analysis was obtained by slow evaporation of the solvent from a diluted solution containing 15 mg of the complex in 2.0 ml of toluene at room temperature. With (R)-BINOL being used as the resolution reagent, the absolute configuration of diketone in the complex was confirmed to be S. The ORTEP drawing of the complex (S)-4·(R)-BINOL is shown in Fig. 2, which clearly shows the inclusion complex containing a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
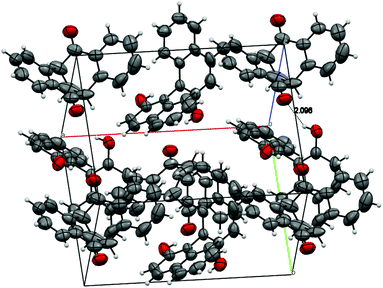
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio of the diketone and BINOL.15 Strong hydrogen bonding was observed, where the nearest distance of two oxygen atoms [HO⋯O
1 molar ratio of the diketone and BINOL.15 Strong hydrogen bonding was observed, where the nearest distance of two oxygen atoms [HO⋯O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C] is 2.827 Å, and the hydrogen bond OH⋯O
C] is 2.827 Å, and the hydrogen bond OH⋯O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C is 2.096 Å (Fig. 3).
C is 2.096 Å (Fig. 3).
The resolution in various solvents was also investigated. With CH2Cl2, ethyl acetate, or mixed PhMe–EtOAc as the solvent, the mass recovery after the precipitation was lower, and as a result the ee of (R)-4 in the mother liquid was significantly decreased (Table 2, entries 1–3). Furthermore, the reaction gave only 22% yield after precipitation when the resolution was conducted in THF, even though the concentration was increased to 0.50 mol L−1 (entry 4). Due to the poor solubility of 4 in t-BuOMe, the resolution was conducted at a diluted condition, which gave a good yield and reasonable enantioselectivity (entry 5).
| Entry | Solvent | Conc. (mol L−1) | Precipitation (S)-4 | Mother liquor (R)-4 | ||
|---|---|---|---|---|---|---|
| Yieldb/% | ee% | Yieldb/% | ee% | |||
| a The reactions were conducted at 1.0 mmol scale of (±)-4. b Yields refer to the isolated yields after the decomposition with aqueous NaOH. c The ee% was not determined. | ||||||
| 1 | CH2Cl2 | 0.20 | 31 | 94 | 67 | 56 |
| 2 | EtOAc | 0.20 | 33 | 90 | 56 | 48 |
| 3 | Toluene–EtOAc (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
0.20 | 40 | 92 | 49 | 58 |
| 4 | THF | 0.50 | 22 | 88 | 77 | —c |
| 5 | t-BuOMe | 0.07 | 44 | 89 | 54 | —c |
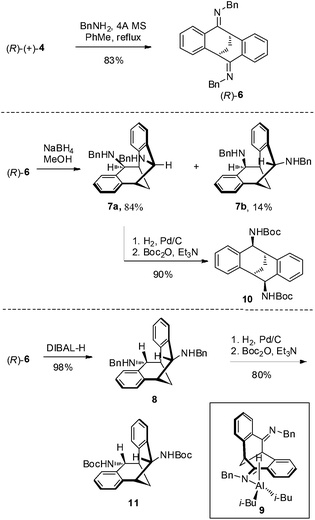
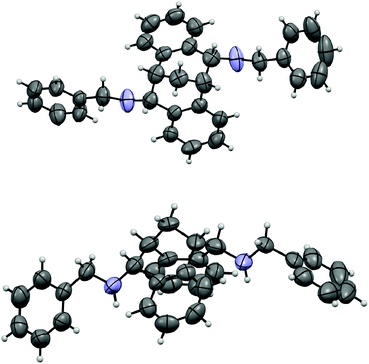
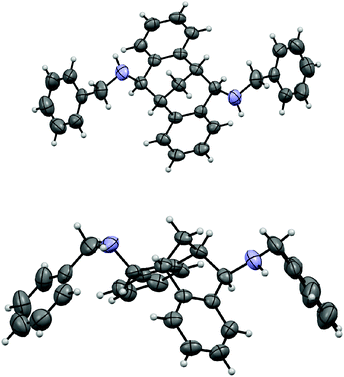
The carbonyl group in 4 provided opportunities for further transformations to other useful chiral compounds. Diketone (R)-(+)-4 was advanced to bis-imine (R)-6 as a single isomer in the presence of benzylamine and 4 Å molecular sieves at reflux in toluene (Scheme 2).16 Following the known procedure, reduction of the imine with NaBH4 in MeOH gave endo-product 7a in good yield, albeit a small amount of other isomers, such as 7b were also detected.12 The selectivity was presumably controlled by steric factors, where the reagent comes from the less hindered convex side of (R)-(+)-6. When diisobutylaluminium hydride (DIBAL-H) was used, the reaction delivered exo-product 8 exclusively in excellent yield. This observation can possibly be explained by model 9 involving a tetrahedral aluminium complex. The sterically bulky isobutyl groups preferred to be at the convex side due to steric hindrance, and as a result the hydride was pushed to be at the concave position to give the exo-product. The related stereochemistry of both diamines 7a17 and 8 was unambiguously confirmed by X-ray crystallographic analysis (Fig. 4 and 5).18 The benzyl groups in 7a and 8 could be de-protected under standard hydrogenation conditions with high selectivity. For the convenience of purification, the diamines were advanced to 10 and 11 in high overall yields.19
In conclusion, we have described a practical and efficient method for enantiomeric resolution of 2,3:6,7-dibenzobicyclo[3.3.1]nona-2,6-diene-4,8-dione (±)-4. Multi-gram quantities of both enantiopure (S)- and (R)-4 could be synthesized from 2-phenylacetonitrile without the need for column chromatography. Further transformations of enantiopure 4 to its diamine derivatives 7a and 8 were also performed. Synthetic applications, molecular recognition, and self-assembly studies of these V-shaped molecules are underway in our laboratory.
This work was financially supported by NSFC (no. 21272221), the Fundamental Research Funds for the Central Universities, and the Recruitment Program of Global Experts. We thank Prof. A. Zakarian (UCSB, USA) for the assistance for manuscript preparation.
Notes and references
- J. Tröger, J. Prakt. Chem., 1887, 36, 225 CrossRef.
- For a review, see: S. Sergeyev, Helv. Chim. Acta, 2009, 92, 415 CrossRef CAS ; For some recent typical results, see: (a) J. Artacho, E. Ascic, T. Rantanen, C.-J. Wallentin, S. Dawaigher, K.-E. Bergquist, M. Harmata, V. Snieckus and K. Wärnmark, Org. Lett., 2012, 14, 4706 CrossRef CAS PubMed; (b) E. B. Veale and T. Gunnlaugsson, J. Org. Chem., 2010, 75, 5513 CrossRef CAS PubMed; (c) C. A. M. Abella, M. Benassi, L. S. Santos, M. N. Eberlin and F. Coelho, J. Org. Chem., 2007, 72, 4048 CrossRef CAS PubMed.
- A very recent example for resolution, see: D. L. Jameson, T. Field, M. R. Schmidt, A. K. DeStefano, C. J. Stiteler, V. J. Venditto, B. Krovic, C. M. Hoffman, M. T. Ondisco and M. E. Belowich, J. Org. Chem., 2013, 78, 11590 CrossRef CAS PubMed , and references cited therein.
- (a) U. Kiehne, T. Weilandt and A. Lützen, Org. Lett., 2007, 9, 1283 CrossRef CAS PubMed; (b) E. M. Boyle, S. Comby, J. K. Molloy and T. Gunnlaugsson, J. Org. Chem., 2013, 78, 8312 CrossRef CAS PubMed; (c) X. Zhu, C.-L. Do-Thanh, C. R. Murdock, K. M. Nelson, C. Tian, S. Brown, S. M. Mahurin, D. M. Jenkins, J. Hu, B. Zhao, H. Liu and S. Dai, ACS Macro Lett., 2013, 2, 660 CrossRef CAS.
- Optically pure porphyrin-Tröger’s base and acridine substituted Tröger’s base were prepared and used in the studies: (a) M. J. Crossley, T. W. Hambley, L. G. Mackay, A. C. Try and R. Walton, J. Chem. Soc., Chem. Commun., 1995, 1077 RSC; (b) A. Tatibouët, M. Demeunyck, C. Andraud, A. Collet and J. Lhomme, Chem. Commun., 1999, 161 RSC.
- (a) A. Greenberg, N. Molinaro and M. Lang, J. Org. Chem., 1984, 49, 1127 CrossRef CAS; (b) D. A. Lenev, K. A. Lyssenko, D. G. Golovanov, V. Buss and R. G. Kostyanovsky, Chem.–Eur. J., 2006, 12, 6412 CrossRef CAS PubMed.
- Synthesis of configurationally stable Tröger’s base, see: (a) Y. Hamada and S. Mukai, Tetrahedron: Asymmetry, 1996, 7, 2671 CrossRef CAS; (b) D. A. Lenev, D. G. Golovanov, K. A. Lyssenko and R. G. Kostyanovsky, Tetrahedron: Asymmetry, 2006, 17, 2191 CrossRef CAS PubMed; (c) C. Michon, A. Sharma, G. Bernardinelli, E. Francotte and J. Lacour, Chem. Commun., 2010, 46, 2206 RSC; (d) A. Sharma, L. Guénée, J.-V. Naubron and J. Lacour, Angew. Chem., Int. Ed., 2011, 50, 3677 CrossRef CAS PubMed.
- J. Kagan, S.-Y. Chen and D. A. Agdeppa Jr., Tetrahedron Lett., 1977, 51, 4469 CrossRef.
- R. S. Prasad and R. M. Roberts, J. Org. Chem., 1991, 56, 2998 CrossRef CAS.
- (a) H. Tatamitsu, F. Ogura, Y. Nakagawa, M. Nanagawa, K. Naemura and M. Nakazaki, Bull. Chem. Soc. Jpn., 1975, 48, 2473 CrossRef; (b) K. Naemura, R. Fukunaga, M. Komatsu, M. Yamanaka and H. Chikamatsu, Bull. Chem. Soc. Jpn., 1989, 62, 83 CrossRef CAS; (c) M. Kimber, A. C. Try, L. Painter, M. M. Harding and P. Turner, J. Org. Chem., 2000, 65, 3042 CrossRef CAS PubMed.
- For the limited applications of Tröger’s base derivatives in organic synthesis, see: (a) M. Harmata and M. Kahraman, Tetrahedron: Asymmetry, 2000, 11, 2875 CrossRef CAS; (b) Y. Shen, M. Zhao, J. Xu and Y. Shi, Angew. Chem., Int. Ed., 2006, 45, 8005 CrossRef CAS PubMed.
- L. Thunberg, S. Allenmark, A. Friberg, F. Ek and T. Frejd, Chirality, 2004, 16, 614 CrossRef CAS PubMed.
- (a) K. Tanaka, T. Okada and F. Toda, Angew. Chem., Int. Ed. Engl., 1993, 32, 1147 CrossRef; (b) F. Toda, K. Tanaka, Z. Stein and I. Goldberg, J. Org. Chem., 1994, 59, 5748 CrossRef CAS; (c) Q.-S. Hu, D. Vitharana and L. Pu, Tetrahedron: Asymmetry, 1995, 6, 2123 CrossRef CAS; (d) D. Cai, D. L. Hughes, T. R. Verhoeven and P. J. Reider, Tetrahedron Lett., 1995, 36, 7991 CrossRef CAS; (e) F. Toda and K. Tanaka, Chem. Commun., 1997, 1087 RSC; (f) K. Ding, Y. Wang, H. Yun, J. Liu, Y. Wu, M. Terada, Y. Okubo and K. Mikami, Chem.–Eur. J., 1999, 5, 1734 CrossRef CAS; (g) J.-H. Zhang, J. Liao, X. Cui, K.-B. Yu, J. Zhu, J.-G. Deng, S.-F. Zhou, L.-X. Wang, Q.-L. Zhou, L. W. Chung and T. Ye, Tetrahedron: Asymmetry, 2002, 13, 1363 CrossRef CAS.
- For a trial of resolution of spiro[4,4]-nonane-1,6-dione by 1,1′-bi-2-naphthol, see: X. Feng, D. Zeng, Z. Li and Y. Jiang, Chin. Chem. Lett., 1999, 10, 559 CAS.
- CCDC 969970 contains the supplementary crystallographic data for complex (S)-4·(R)-BINOL.
- The geometry of the bis-imine was confirmed by 1H NMR and NOE studies, see ESI† for details.
- CCDC 969971 contain the supplementary crystallographic data for compound rac-7a.
- CCDC 969972 contain the supplementary crystallographic data for compound rac-8.
- A pair of rotamers were observed by 1H NMR at room temperature for both 10 and 11 probably due to the partially inhibited rotation of the amide functionalities. Clean NMR spectra could be obtained by taking the NMR spectra at elevated temperature, see ESI† for details.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures, characterization data, CIF files for (S)-4·(R)-BINOL, rac-7a and rac-8, 1H and 13C NMR spectra for all new compounds. CCDC 969970–969972. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c4qo00011k |
| This journal is © the Partner Organisations 2014 |