Regiocontrolled 1,2-migration in cyclization of 1-(indol-2-yl)-3-alkyn-1-ols: (Ph3P)Au+vs. PtCl4†
Youai
Qiu
,
Dengke
Ma
,
Wangqing
Kong
,
Chunling
Fu
and
Shengming
Ma
*
Laboratory of Molecular Recognition and Synthesis, Department of Chemistry, Zhejiang University, 310027 Hangzhou, Zhejiang, P. R. China. E-mail: masm@sioc.ac.cn; Fax: (+86) 21-62609305
First published on 20th December 2013
Abstract
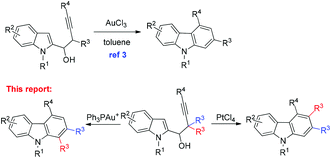
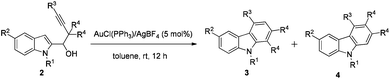
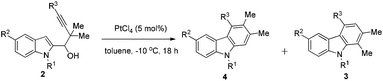
An AuCl(PPh3)/AgBF4- and PtCl4-catalyzed reaction of 1-(indol-2-yl)-3-alkyn-1-ols occurred smoothly in toluene to form a series of differently polysubstituted carbazole derivatives efficiently. The regioselectivity of the 1,2-migration may be tuned by using different metal catalysts: carbazoles 3 could be obtained exclusively in the presence of AuCl(PPh3)/AgBF4via a Wagner–Meerwein type 1,2-alkyl shift, whereas in some cases the use of PtCl4 afforded differently substituted carbazoles 4 involving a platinum–carbene intermediate.
Introduction
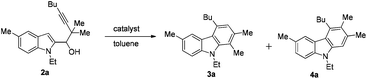
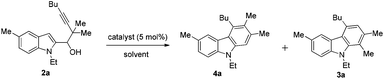
Control of selectivity remains one of the most important challenges in organic chemistry.1 Among the many strategies employed, catalyst-based control of selectivity2 has been proven to be very beneficial for the simple reason that the same substrates are applied for different products. Recently, we have described an approach to the carbazole skeleton through a AuCl3-catalyzed cyclization of 1-(indol-2-yl)-3-alkyn-1-ols.3 With such simple substrates, the mechanism of such a transformation remains a puzzle. With the purpose of mechanistic study and extending the scope of this reaction, we prepared 2,2-dimethyl-substituted substrate 2a.4Quite unexpectedly, carbazoles 3a and 4a with one of the two methyl groups in the 1- or 3-position of the carbazole skeleton were formed in a ratio of 3a/4a = 34/18 (entry 1, Table 1). Based on these stimulating results, we were interested in the pathway(s) for the formation of both products and envisioned that if such a regioselectivity may be controlled, it would provide a highly selective entry to different carbazoles5 from the same substrates. In this paper, we wish to report the realization of such a concept (Scheme 1).Results and discussion
Studies were conducted to optimize the conditions to improve the selectivity of 3a and 4a with some typical results listed in Table 1. We attempted to apply the cationic gold catalyst6 first by the addition of different Ag+ salts to AuCl(PPh3): interestingly, the yield of 3a was improved to 77% with AgSbF6 as the co-catalyst (entry 2, Table 1);7 screening of other silver salts led to the observation that AgBF4 is the best (entry 5, Table 1); no better results were obtained at a lower temperature (entries 6 and 7, Table 1); (IPr)AuCl (IPr = N,N′-bis(2,6-diisopropylphenyl)imidazol-2-ylidene)/AgBF4 may catalyze this transformation affording 3a in 55% yield together with 4a in 9% yield (entry 8, Table 1); interestingly, InBr3 also afforded a moderate yield of 3a with 94![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 selectivity (entry 9, Table 1).
6 selectivity (entry 9, Table 1).
| Entry | Catalyst (5 mol%) | Temp. (°C) | Time (h) | Yieldb (%) |
3a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4ab 4ab |
|
|---|---|---|---|---|---|---|
| 3a | 4a | |||||
| a The reaction was conducted with 0.2 mmol of 2a and 5 mol% catalyst in 2 mL of toluene. b Determined by NMR using dibromomethane as the internal standard. | ||||||
| 1 | AuCl3 | rt | 42 | 34 | 18 | 65![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 35 35 |
| 2 | AuCl(PPh3)/AgSbF6 | rt | 12 | 77 | 8 | 91![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 9 |
| 3 | AuCl(PPh3)/AgOTf | rt | 12 | 81 | 3 | 96![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
| 4 | AuCl(PPh3)/AgPF6 | rt | 12 | 77 | 4 | 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5 |
| 5 | AuCl(PPh3)/AgBF4 | rt | 12 | 82 | 2 |
98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 2 2
|
| 6 | AuCl(PPh3)/AgBF4 | 10 | 5 | 74 | 2 | 97![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
| 7 | AuCl(PPh3)/AgBF4 | 5 | 5 | 54 | 2 | 96![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
| 8 | (IPr)AuCl/AgBF4 | rt | 12 | 55 | 9 | 86![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 14 14 |
| 9 | InBr3 | 80 | 12 | 67 | 4 | 94![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 6 |
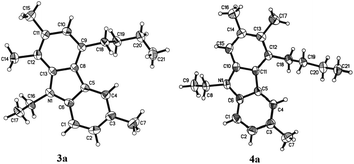
The structures of 3a and 4a have been unambiguously determined by their X-ray single crystal diffraction study (Fig. 1).8
The scope was then explored with the optimized Au-catalyzed conditions: the 1-position of indoles may be substituted with various alkyl groups, such as methyl, ethyl, butyl, or even an allyl group. Substituents on the 5-position could be methyl (entries 1, 5, and 8, Table 2), methoxy (entry 3, Table 2) or bromo, which could easily be transformed to some useful functional groups in the synthesis of biologically active carbazole alkaloids9 (entry 4, Table 2). R3 could be an alkyl (entries 1–7 and 9, Table 2) or a phenyl group (entry 8, Table 2), although the selectivity for the substrate with the phenyl group 2h is slightly lower (93![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7). The reaction can be easily conducted on a scale of 3.5 mmol of 2b (1.0401 g) in a slightly higher yield (entry 9, Table 2). We observed that ethyl may also migrate exclusively in good selectivity (entry 10, Table 2). Moreover, from the practical synthetic viewpoint, pure carbazoles 3 could be afforded by simple recrystallization (entries 1–5 and 7–9, Table 2).
7). The reaction can be easily conducted on a scale of 3.5 mmol of 2b (1.0401 g) in a slightly higher yield (entry 9, Table 2). We observed that ethyl may also migrate exclusively in good selectivity (entry 10, Table 2). Moreover, from the practical synthetic viewpoint, pure carbazoles 3 could be afforded by simple recrystallization (entries 1–5 and 7–9, Table 2).
| Entry | 2 | Yield (3/4)b,c (%) | Yield of 3d (%) |
|---|---|---|---|
| R1/R2/R3/R4 | |||
a The reaction was conducted with 1.0 mmol of 2a and 5 mol% AuCl(PPh3)/AgBF4 in 10 mL of toluene at room temperature.
b Isolated yield of 3 and 4.
c The ratio of 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 is presented in parentheses.
d Yield of 3 after recrystallization.
e The reaction was completed in 18 h.
f 10 mol% AuCl(PPh3)/AgBF4 was used.
g The reaction was conducted with a 3.5 mmol (1.0401 g) scale of 2b. 4 is presented in parentheses.
d Yield of 3 after recrystallization.
e The reaction was completed in 18 h.
f 10 mol% AuCl(PPh3)/AgBF4 was used.
g The reaction was conducted with a 3.5 mmol (1.0401 g) scale of 2b.
|
|||
| 1 | Et/Me/Bu/Me (2a) | 64 (97![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) (3a:4a) 3) (3a:4a) |
52 |
| 2 | Et/H/Bu/Me (2b) | 64 (97![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) (3b:4b) 3) (3b:4b) |
45 |
| 3 | Et/OMe/Bu/Me (2c) | 68 (97![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) (3c:4c) 3) (3c:4c) |
58 |
| 4 | Me/Br/Bu/Me (2d) | 55 (97![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) (3d:4d) 3) (3d:4d) |
47 |
| 5 | Me/Me/Bu/Me (2e) | 72 (97![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) (3e:4e) 3) (3e:4e) |
59 |
| 6e | Bu/H/Bu/Me (2f) | 67 (97![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) (3f:4f) 3) (3f:4f) |
— |
| 7 | Allyl/H/Bu/Me (2g) | 69 (96![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) (3g:4g) 4) (3g:4g) |
54 |
| 8e,f | Et/Me/Ph/Me (2h) | 67 (93![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7) (3h:4h) 7) (3h:4h) |
57 |
| 9g | Et/H/Bu/Me (2b) | 66 (97![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) (3b:4b) 3) (3b:4b) |
51 |
| 10 | Et/Me/Bu/Et (2i) | 57 (98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) (3i:4i) 2) (3i:4i) |
— |
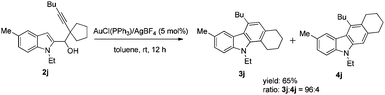
In addition, we have studied the reaction of the substrate with a 5-membered cycle 2j, which underwent a smooth cyclization with ring expansion to afford fused tetracyclic carbazole 3j in 65% yield (eqn (1)).
 | (1) |
In order to invert the selectivity with the purpose of highly selective formation of carbazole 4a, different metal catalysts were then screened: AuCl, AgBF4 or Cu(OTf)2 failed to promote the reaction smoothly (entries 1–3, Table 3); it is exciting to observe an inverted regioselectivity when PtCl2 was employed as the catalyst (entry 4, Table 3). In contrast to several Pt(II) catalysts (entries 4–8, Table 3), the higher oxidation state of the platinum catalyst, i.e., PtCl4, shows a better selectivity (entry 9, Table 3). Several solvents were then tested for the PtCl4-catalyzed reaction of 2a at room temperature with toluene still being the best (entries 9–13, Table 3). Furthermore, the effect of temperature was considered (entries 9 and 14–16, Table 3): the best yield (82%) and selectivity (4a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3a = 93
3a = 93![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7) were realized at −10 °C (entry 15, Table 3). Interestingly, the ratio of 4a
7) were realized at −10 °C (entry 15, Table 3). Interestingly, the ratio of 4a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3a dropped again at −20 °C (entry 16, Table 3).
3a dropped again at −20 °C (entry 16, Table 3).
| Entry | Catalyst | Temp. (°C) | Solvent | Time (h) | Yieldb (%) |
4a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3ab 3ab |
|
|---|---|---|---|---|---|---|---|
| 4a | 3a | ||||||
| a The reaction was conducted with 0.2 mmol of 2a and 5 mol% catalyst in 2 mL of solvent. b Determined by NMR using dibromomethane as the internal standard. c The recovery of 2a was 53%. d The recovery of 2a was 65%. e The recovery of 2a was 100%. f The recovery of 2a was 94%. g The recovery of 2a was 92%. | |||||||
| 1c | AuCl | rt | Toluene | 18 | 5 | 13 | 28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 72 72 |
| 2d | AgBF4 | rt | Toluene | 13 | — | 4 | — |
| 3 | Cu(OTf)2 | 80 | Toluene | 12 | 1 | 8 | 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 89 89 |
| 4 | PtCl2 | rt | Toluene | 18 | 71 | 15 | 83![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 17 17 |
| 5e | PtI2 | rt | Toluene | 12 | — | — | — |
| 6 | PtI2 | 80 | Toluene | 12 | 72 | 19 | 79![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 21 21 |
| 7f | Pt(CN)2 | rt | Toluene | 24 | — | — | — |
| 8g | PtCl2(PhCN)2 | rt | Toluene | 18 | — | — | — |
| 9 | PtCl4 | rt | Toluene | 18 | 74 | 11 | 87![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 13 13 |
| 10 | PtCl4 | rt | DCM | 5 | 24 | 4 | 86![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 14 14 |
| 11 | PtCl4 | rt | Xylenes | 8 | 73 | 12 | 86![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 14 14 |
| 12 | PtCl4 | rt | THF | 8 | 26 | 26 | 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 50 |
| 13 | PtCl4 | rt | Dioxane | 8 | 53 | 27 | 66![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 34 34 |
| 14 | PtCl4 | −5 | Toluene | 18 | 77 | 10 | 89![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11 11 |
| 15 | PtCl4 | −10 | Toluene | 18 | 81 | 6 |
93![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 7 7
|
| 16 | PtCl4 | −20 | Toluene | 18 | 70 | 7 | 91![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 9 |
Under the PtCl4-catalyzed conditions (entry 15, Table 3), the reversed regioselective 1,2-methyl migration10 was then explored: R1 could be a series of alkyl groups, such as methyl, ethyl, and butyl. Substituents on the 5-position of indoles could be methyl (entries 1, 4, and 6, Table 4) and methoxy (entry 3, Table 4). R3 could be an alkyl (entries 1–5, Table 4) or a phenyl group (entry 6, Table 4). Moreover, again from the practical synthetic viewpoint, the ratio of 4 and 3 could be improved by simple recrystallization (entries 1, 3, and 6, Table 4).
| Entry | 2 | Yield (4/3)b,c (%) | Yield and ratiod (%) |
|---|---|---|---|
| R1/R2/R3 | |||
a The reaction was conducted with 1.0 mmol of 2a and 5 mol% PtCl4 in 10 mL of toluene at −10 °C.
b Isolated yield of 4 and 3.
c The ratio of 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 is presented in parentheses.
d Yield and ratio after recrystallization.
e The reaction was completed in 12 h.
f The reaction was completed in 72 h. 3 is presented in parentheses.
d Yield and ratio after recrystallization.
e The reaction was completed in 12 h.
f The reaction was completed in 72 h.
|
|||
| 1 | Et/Me/Bu (2a) | 80 (92![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8) (4a 8) (4a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3a) 3a) |
61 (96![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) 4) |
| 2 | Et/H/Bu (2b) | 81 (90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10) (4b 10) (4b![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3b) 3b) |
— |
| 3 | Et/OMe/Bu (2c) | 80 (89![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11) (4c 11) (4c![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3c) 3c) |
65 (98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) 2) |
| 4e | Me/Me/Bu (2e) | 77 (91![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9) (4e 9) (4e![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3e) 3e) |
— |
| 5 | Bu/H/Bu (2f) | 78 (89![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11) (4f 11) (4f![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3f) 3f) |
— |
| 6f | Et/Me/Ph (2h) | 78 (93![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7) (4h 7) (4h![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3h) 3h) |
61 (97![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) 3) |
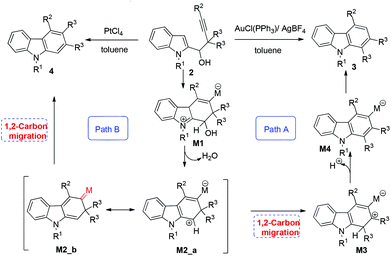
With this information, a rationale was proposed for this reaction (Scheme 2). The reaction of Au or Pt with 2 would form intermediate M1via the coordination of the alkyne with the gold or platinum atom followed by a nucleophilic attack of indolyl C3 to these metal-activated C–C triple bonds.11 The intermediate M2_a may be afforded via protonation of the hydroxyl group in M1 followed by elimination of H2O.12 The R3 group subsequently migrates to the carbocationic center to form a new carbocationic intermediate M3.13 Subsequent elimination of H+ of M3 would afford intermediate M4. Finally, protonolysis would release the gold catalyst into the catalytic cycle to afford the target carbazole 3 (path A, Scheme 2). With PtCl4 as the catalyst, the reaction proceeds via the resonance structure vinylic platinum carbene (M2_b), affording the final product 4via 1,2-alkyl migration14 (path B, Scheme 2). The real role of each catalyst for the different selectivity is still not clear.
In conclusion, we have developed a simple and efficient AuCl(PPh3)/AgBF4- or PtCl4-catalyzed reaction of 1-(indol-2-yl)-2,2-dialkyl-substituted-3-alkyne-1-ols, providing differently substituted carbazoles in good isolated yields under very mild conditions. Different regioselective 1,2-alkyl migration pathways have been established: carbazoles 3 could be obtained exclusively in the presence of AuCl(PPh3)/AgBF4via a Wagner–Meerwein type 1,2-alkyl shift, whereas in some cases the use of PtCl4 afforded inverted regioselectivity forming carbazoles 4 involving a platinum–carbene intermediate. Due to the potential of the products and unique pathways, this method may be useful in organic synthesis and medicinal chemistry. The observed selectivity with different metal catalysts is quite informative for further study. Further studies including new ways for the synthesis of the starting materials and synthetic applications of this reaction and the effect of the catalyst on the selectivity are being carried out in our laboratory.
Experimental
1. AuCl(PPh3)/AgBF4-catalyzed cyclization reaction of 1-(indol-2-yl)-2,2-dialkyl-3-alkyne-1-ols: synthesis of 4-butyl-9-ethyl-1,2,6-trimethyl-9H-carbazole (3a)
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4a = 97
4a = 97![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 determined by 1H NMR of crude product, column chromatography on silica gel (petroleum ether–dichloromethane = 30/l for the first round, petroleum ether–dichloromethane = 30/l for the second round (impure part)) afforded 3a (188.1 mg, 64%, 3a
3 determined by 1H NMR of crude product, column chromatography on silica gel (petroleum ether–dichloromethane = 30/l for the first round, petroleum ether–dichloromethane = 30/l for the second round (impure part)) afforded 3a (188.1 mg, 64%, 3a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4a = 97
4a = 97![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3), which was further purified by recrystallization to afford pure 3a (152.8 mg, 52%) as a solid: m.p. 80–82 °C (n-hexane–ethyl acetate); 1H NMR (300 MHz, CDCl3) δ 7.94 (s, 1H, ArH), 7.34 (d, J = 8.4 Hz, 1H, ArH), 7.31 (d, J = 8.4 Hz, 1H, ArH), 6.92 (s, 1H, ArH), 4.61 (q, J = 7.1 Hz, 2H, NCH2), 3.22 (t, J = 7.8 Hz, 2H, ArCH2), 2.73 (s, 3H, CH3), 2.62 (s, 3H, CH3), 2.53 (s, 3H, CH3), 1.96–1.82 (m, 2H, CH2), 1.71–1.58 (m, 2H, CH2), 1.48 (t, J = 6.9 Hz, 3H, CH3), 1.10 (t, J = 7.4 Hz, 3H, CH3); 13C NMR (75 MHz, CDCl3) δ 140.3, 139.7, 135.2, 134.2, 127.9, 125.7, 123.4, 122.7, 122.4, 120.0, 115.7, 108.2, 39.9, 33.9, 31.9, 23.0, 21.6, 20.9, 15.5, 14.8, 14.1; IR (KBr) ν (cm−1) 3011, 2955, 2928, 2862, 1592, 1574, 1484, 1377, 1343, 1308, 1229, 1172, 1150, 1081; MS (70 ev, EI) m/z (%) 294 (M+ + 1, 21.92), 293 (M+, 100); elemental analysis calcd (%) for C21H27N: C, 85.95; H, 9.27; N, 4.77; found: C, 85.64, H, 9.31; N, 4.84.
3), which was further purified by recrystallization to afford pure 3a (152.8 mg, 52%) as a solid: m.p. 80–82 °C (n-hexane–ethyl acetate); 1H NMR (300 MHz, CDCl3) δ 7.94 (s, 1H, ArH), 7.34 (d, J = 8.4 Hz, 1H, ArH), 7.31 (d, J = 8.4 Hz, 1H, ArH), 6.92 (s, 1H, ArH), 4.61 (q, J = 7.1 Hz, 2H, NCH2), 3.22 (t, J = 7.8 Hz, 2H, ArCH2), 2.73 (s, 3H, CH3), 2.62 (s, 3H, CH3), 2.53 (s, 3H, CH3), 1.96–1.82 (m, 2H, CH2), 1.71–1.58 (m, 2H, CH2), 1.48 (t, J = 6.9 Hz, 3H, CH3), 1.10 (t, J = 7.4 Hz, 3H, CH3); 13C NMR (75 MHz, CDCl3) δ 140.3, 139.7, 135.2, 134.2, 127.9, 125.7, 123.4, 122.7, 122.4, 120.0, 115.7, 108.2, 39.9, 33.9, 31.9, 23.0, 21.6, 20.9, 15.5, 14.8, 14.1; IR (KBr) ν (cm−1) 3011, 2955, 2928, 2862, 1592, 1574, 1484, 1377, 1343, 1308, 1229, 1172, 1150, 1081; MS (70 ev, EI) m/z (%) 294 (M+ + 1, 21.92), 293 (M+, 100); elemental analysis calcd (%) for C21H27N: C, 85.95; H, 9.27; N, 4.77; found: C, 85.64, H, 9.31; N, 4.84.
2. PtCl4-catalyzed cyclization reaction of 1-(indol-2-yl)-2,2-dimethyl-3-alkyne-1-ols: synthesis of 4-butyl-9-ethyl-2,3,6-trimethyl-9H-carbazole (4a)
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3a = 92
3a = 92![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 determined by 1H NMR of crude product, and column chromatography on silica gel (petroleum ether–ethyl acetate = 100/l) afforded 4a and 3a (235.0 mg, 80%, 4a
8 determined by 1H NMR of crude product, and column chromatography on silica gel (petroleum ether–ethyl acetate = 100/l) afforded 4a and 3a (235.0 mg, 80%, 4a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3a = 92
3a = 92![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 determined by 1H NMR), which was further purified by recrystallization to afford 4a (179.3 mg, 61%, 4a
8 determined by 1H NMR), which was further purified by recrystallization to afford 4a (179.3 mg, 61%, 4a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3a = 96
3a = 96![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) as a solid: m.p. 84–86 °C (n-hexane/ethyl acetate); 1H NMR of 4a (300 MHz, CDCl3) δ 7.99 (s, 1H, ArH), 7.37–7.27 (m, 2H, ArH), 7.14 (s, 1H, ArH), 4.34 (q, J = 7.1 Hz, 2H, NCH2), 3.33 (t, J = 8.0 Hz, 2H, ArCH2), 2.62 (s, 3H, ArCH3), 2.56 (s, 3H, ArCH3), 2.43 (s, 3H, ArCH3), 1.92–1.62 (m, 4H, 2 × CH2), 1.44 (t, J = 7.1 Hz, 3H, CH3), 1.13 (t, J = 7.2 Hz, 3H, CH3); the following signals are discernible for 3a: 7.93 (s, 1H, ArH), 6.91 (s, 1H, ArH), 4.60 (q, J = 7.1 Hz, 2H, NCH2), 3.21 (t, J = 7.7 Hz, 2H, ArCH2), 2.73 (s, 3H, ArCH3); 13C NMR of 4a (75 MHz, CDCl3) δ 138.8, 138.2, 136.4, 134.8, 127.4, 125.4, 124.6, 123.2, 122.4, 119.1, 107.7, 107.1, 37.1, 31.4, 30.3, 23.4, 22.3, 21.7, 14.5, 14.1, 13.6; IR (neat) ν (cm−1) 2956, 2929, 2871, 1623, 1605, 1576, 1487, 1471, 1377, 1349, 1307, 1266, 1192, 1147, 1015; GC-MS (GC conditions: injector: 280 °C; column: DB5 column 30 m × 0.25 mm, temperature programming: 60 °C (2 min), 20 °C min−1 to 280 °C, 280 °C (30 min); detector: 280 °C) (70 ev, EI) m/z (%) for 4a: TR 5.290 min: 294 (M+ + 1, 22.77), 293 (M+, 100), for 3a: TR 5.313 min: 294 (M+ + 1, 23.31), 293 (M+, 100); elemental analysis calcd (%) for C21H27N: C, 85.95; H, 9.27; N, 4.77; found: C, 85.91; H, 9.46; N, 4.91.
4) as a solid: m.p. 84–86 °C (n-hexane/ethyl acetate); 1H NMR of 4a (300 MHz, CDCl3) δ 7.99 (s, 1H, ArH), 7.37–7.27 (m, 2H, ArH), 7.14 (s, 1H, ArH), 4.34 (q, J = 7.1 Hz, 2H, NCH2), 3.33 (t, J = 8.0 Hz, 2H, ArCH2), 2.62 (s, 3H, ArCH3), 2.56 (s, 3H, ArCH3), 2.43 (s, 3H, ArCH3), 1.92–1.62 (m, 4H, 2 × CH2), 1.44 (t, J = 7.1 Hz, 3H, CH3), 1.13 (t, J = 7.2 Hz, 3H, CH3); the following signals are discernible for 3a: 7.93 (s, 1H, ArH), 6.91 (s, 1H, ArH), 4.60 (q, J = 7.1 Hz, 2H, NCH2), 3.21 (t, J = 7.7 Hz, 2H, ArCH2), 2.73 (s, 3H, ArCH3); 13C NMR of 4a (75 MHz, CDCl3) δ 138.8, 138.2, 136.4, 134.8, 127.4, 125.4, 124.6, 123.2, 122.4, 119.1, 107.7, 107.1, 37.1, 31.4, 30.3, 23.4, 22.3, 21.7, 14.5, 14.1, 13.6; IR (neat) ν (cm−1) 2956, 2929, 2871, 1623, 1605, 1576, 1487, 1471, 1377, 1349, 1307, 1266, 1192, 1147, 1015; GC-MS (GC conditions: injector: 280 °C; column: DB5 column 30 m × 0.25 mm, temperature programming: 60 °C (2 min), 20 °C min−1 to 280 °C, 280 °C (30 min); detector: 280 °C) (70 ev, EI) m/z (%) for 4a: TR 5.290 min: 294 (M+ + 1, 22.77), 293 (M+, 100), for 3a: TR 5.313 min: 294 (M+ + 1, 23.31), 293 (M+, 100); elemental analysis calcd (%) for C21H27N: C, 85.95; H, 9.27; N, 4.77; found: C, 85.91; H, 9.46; N, 4.91.
Acknowledgements
Financial support from the National Natural Science Foundation of China (21232006) and the National Basic Research Program (2011CB808700) is greatly appreciated. Shengming Ma is a Qiu Shi Adjunct Professor at Zhejiang University. We thank Mr X. Tang of this group for reproducing the preparation of 3e and 3g in Table 2 and 4e in Table 4.Notes and references
- For selected reviews, see: (a) S. R. Neufelst and M. S. Sanford, Acc. Chem. Res., 2012, 45, 936 CrossRef PubMed; (b) N. Krause and C. Winter, Chem. Rev., 2011, 111, 1994 CrossRef CAS PubMed; (c) E. Soriano and J. Marco-Contelles, Acc. Chem. Res., 2009, 42, 1026 CrossRef CAS PubMed; (d) M. T. Whited and R. H. Grubbs, Acc. Chem. Res., 2009, 42, 1607 CrossRef CAS PubMed; (e) B. Crone and S. F. Kirsch, Chem.–Eur. J., 2008, 14, 3514 CrossRef CAS PubMed.
- For selected examples, see: (a) C. Ferrer and A. M. Echavarren, Angew. Chem., Int. Ed., 2006, 45, 1105 CrossRef CAS PubMed; (b) S. G. Modha, A. Kumar, D. D. Vachhami, S. K. Sharma, V. S. Parmar and E. V. V. Eychen, Chem. Commun., 2012, 10916 RSC; (c) L. Zhang, J. Am. Chem. Soc., 2005, 127, 16804 CrossRef CAS PubMed; (d) G. Zhang, V. J. Catalano and L. Zhang, J. Am. Chem. Soc., 2007, 129, 11358 CrossRef CAS PubMed; (e) W. Li, Y. Li, G. Zhou, Z. Wu and J. Zhang, Chem.–Eur. J., 2012, 18, 15113 CrossRef CAS PubMed; (f) T. Sueda, A. Kawada, Y. Urashi and N. Teno, Org. Lett., 2013, 15, 1560 CrossRef CAS PubMed; (g) A. S. K. Hashmi, E. KurPejović, W. Frey and J. W. Bats, Tetrahedron, 2007, 63, 5879 CrossRef CAS; (h) E. Álvarez, P. García-García, M. A. Fernández-Rodríguez and R. Sanz, J. Org. Chem., 2013, 78, 9758 CrossRef PubMed; (i) L. Wang, G. Li and Y. Liu, Org. Lett., 2011, 13, 3786 CrossRef CAS PubMed.
- Y. Qiu, W. Kong, C. Fu and S. Ma, Org. Lett., 2012, 14, 6198 CrossRef CAS PubMed . For a recent similar study, see: Z. Zhang, X. Tang, Q. Xu and M. Shi, Chem.–Eur. J., 2013, 19, 10625 CrossRef PubMed.
- (a) P. H. Lee, H. Kim and K. Lee, Adv. Synth. Catal., 2005, 347, 1219 CrossRef CAS; (b) R. L. Patman, V. M. Williams, J. F. Bower and M. J. Krische, Angew. Chem., Int. Ed., 2008, 47, 5220 CrossRef CAS PubMed; (c) Y. Masuyama, A. Watabe and Y. Kurusu, Synlett, 2003, 1713 CrossRef CAS.
- For reviews on the synthesis of carbazoles, see: (a) H.-J. Knölker and K. R. Reddy, Chem. Rev., 2002, 102, 4303 CrossRef; (b) N. Campbell and B. M. Barclay, Chem. Rev., 1947, 40, 359 CrossRef CAS PubMed; (c) J. Bergman and B. Pelcman, Pure Appl. Chem., 1990, 62, 1967 CrossRef CAS; (d) C. J. Moody, Synlett, 1994, 681 CrossRef CAS; (e) B. C. G. Söderberg, Curr. Org. Chem., 2000, 4, 727 CrossRef; (f) H.-J. Knölker, Chem. Soc. Rev., 1999, 28, 151 RSC; (g) H.-J. Knölker, Top. Curr. Chem., 2005, 244, 115 Search PubMed; (h) U. Pindur and H. Erfanian-Abdoust, Chem. Rev., 1989, 89, 1681 CrossRef CAS.
- For selected reviews on the chemistry of the gold-catalyzed reaction, see: (a) N. D. Shapiro and F. D. Toste, Synlett, 2010, 675 CAS; (b) A. S. K. Hashmi, Angew. Chem., Int. Ed., 2010, 49, 5232 CrossRef CAS PubMed; (c) A. S. K. Hashmi, Chem. Rev., 2007, 107, 2180 CrossRef PubMed; (d) A. Fürstner, Chem. Soc. Rev., 2009, 38, 3208 RSC; (e) D. J. Gorin, B. D. Sherry and F. D. Toste, Chem. Rev., 2008, 108, 3351 CrossRef CAS PubMed; (f) S. F. Krisch, Synthesis, 2008, 3183 CrossRef; (g) Z. Li, C. Brouwer and C. He, Chem. Rev., 2008, 108, 3239 CrossRef CAS PubMed; (h) D. J. Gorin and F. D. Toste, Nature, 2007, 446, 395 CrossRef CAS PubMed; (i) A. S. K. Hashmi and G. J. Hutchings, Angew. Chem., Int. Ed., 2006, 45, 7896 CrossRef PubMed; (j) A. S. K. Hashmi, Top. Organomet. Chem., 2013, 44, 143 CrossRef.
- S. Samala, A. K. Mandadapu, M. Saifuddin and B. Kundu, J. Org. Chem., 2013, 78, 6769 CrossRef CAS PubMed.
- Crystal data for compound 3a: C21H27N: MW = 293.44, monoclinic, space group Pbca, final R indices [I > 2σ(I)], R1 = 0.0479, wR2 = 0.1320; R indices (all data), R1 = 0.0676, wR2 = 0.1184; a = 9.2391(3) Å, b = 17.1518(6) Å, c = 22.2035(6) Å, α = 90.00°, β = 90.00°, γ = 90.00°, V = 3518.53(19) Å3, T = 293(2) K, Z = 8, reflections collected/unique 21053/3218 (Rint = 0.0352), number of observations [>2σ(I)] 2414, parameters: 203. Supplementary crystallographic data have been deposited at the Cambridge Crystallographic Data Centre, CCDC 944138. Crystal data for compound 4a: C21H27N, MW = 293.44, monoclinic, space group P21/c, final R indices [I > 2σ(I)], R1 = 0.0533, wR2 = 0.1538; R indices (all data), R1 = 0.0875, wR2 = 0.1332; a = 13.1716(7) Å, b = 8.3809(5) Å, c = 20.3900(12) Å, α = 90.00°, β = 127.363(4)°, γ = 90.00°, V = 1789.0(2) Å3, T = 293(2) K, Z = 4, reflections collected/unique 7221/3258 (Rint = 0.0252), number of observations [>2σ(I)] 2138, parameters: 204. Supplementary crystallographic data have been deposited at the Cambridge Crystallographic Data Centre, CCDC 944139.
- A. W. Schmidt, K. R. Reddy and H.-J. Knölker, Chem. Rev., 2012, 112, 3193 CrossRef CAS PubMed.
- Selected reports on the 1,2-migration of Au- or Pt-catalyzed reactions, see: (a) W. Li, Y. Li, G. Zhou, Z. Wu and J. Zhang, Chem.–Eur. J., 2012, 18, 15113 CrossRef CAS PubMed; (b) M. R. Luzung, J. P. Markham and F. D. Toste, J. Am. Chem. Soc., 2004, 126, 10858 CrossRef CAS PubMed; (c) M. Méndez, M. P. Muñoz, C. Nevado, D. J. Cárdenas and A. M. Echavarren, J. Am. Chem. Soc., 2001, 123, 10511 CrossRef; (d) L. Zhang and S. A. Kozimin, J. Am. Chem. Soc., 2004, 126, 11806 CrossRef CAS PubMed; (e) J. Sun, M. P. Conley, L. Zhang and S. A. Kozimin, J. Am. Chem. Soc., 2006, 128, 9705 CrossRef CAS PubMed; (f) A. S. K. Hashmi, L. Schwarz, J.-H. Choi and T. M. Frost, Angew. Chem., Int. Ed., 2000, 39, 2285 CrossRef CAS; (g) J. T. Kim, A. V. Kel'in and V. Gevorgyan, Angew. Chem., Int. Ed., 2003, 42, 98 CrossRef CAS; (h) A. W. Sromek, M. Rubina and V. Gevorgyan, J. Am. Chem. Soc., 2005, 127, 10500 CrossRef CAS PubMed; (i) H. Funami, H. Kusama and N. Iwasawa, Angew. Chem., Int. Ed., 2007, 46, 909 CrossRef CAS PubMed; (j) J. H. Lee and F. D. Toste, Angew. Chem., Int. Ed., 2007, 46, 912 CrossRef CAS PubMed; (k) A. S. Dudnik and V. Gevorgyan, Angew. Chem., Int. Ed., 2007, 46, 5195 CrossRef CAS PubMed; (l) A. S. Dudnik, Y. Xia, Y. Li and V. Gevorgyan, J. Am. Chem. Soc., 2010, 132, 7645 CrossRef CAS PubMed.
- For intramolecular hydroarylation of alkynes see: (a) S. J. Pastine, S. W. Youn and D. Sames, Org. Lett., 2003, 5, 1055 CrossRef CAS PubMed; (b) S. J. Pastine, S. W. Youn and D. Sames, Tetrahedron, 2003, 59, 8859 CrossRef CAS; (c) S. J. Pastine and D. Sames, Org. Lett., 2003, 5, 4053 CrossRef CAS PubMed; (d) X.-Y. Liu, P. Ding, J.-S. Huang and C.-M. Che, Org. Lett., 2007, 9, 2645 CrossRef CAS PubMed; (e) R. S. Menon, A. D. Findlay, A. C. Bissember and M. G. Banwell, J. Org. Chem., 2009, 74, 8901 CrossRef CAS PubMed; (f) H. A. Wegner, S. Ahles and M. Neuburger, Chem.–Eur. J., 2008, 14, 11310 CrossRef CAS PubMed; (g) I. D. Jurberg and F. Gagosz, J. Organomet. Chem., 2011, 696, 37 CrossRef CAS; (h) C. Gronnier, Y. Odabachian and F. Gagosz, Chem. Commun., 2011, 47, 218 RSC; (i) D. J. Gorin, P. Dubé and F. D. Toste, J. Am. Chem. Soc., 2006, 128, 14480 CrossRef CAS PubMed; (j) J.-J. Lian, P.-C. Chen, Y.-P. Lin, H.-C. Ting and R.-S. Liu, J. Am. Chem. Soc., 2006, 128, 11372 CrossRef CAS PubMed; (k) C. Nevado and A. M. Echavarren, Chem.–Eur. J., 2005, 11, 3155 CrossRef CAS PubMed; (l) A. S. K. Hashmi, W. Yang and F. Rominger, Angew. Chem., Int. Ed., 2011, 50, 5762 CrossRef CAS PubMed; (m) A. S. K. Hashmi, W. Yang and F. Rominger, Chem.–Eur. J., 2012, 18, 6576 CrossRef CAS PubMed; (n) A. S. K. Hashmi, W. Yang and F. Rominger, Adv. Synth. Catal., 2012, 354, 1273 CrossRef CAS.
- (a) X. Haung and L. Zhang, Org. Lett., 2007, 9, 4627 CrossRef PubMed; (b) A. S. K. Hashmi and M. Wölfle, Tetrahedron, 2009, 65, 9021 CrossRef CAS; (c) W. Kong, C. Fu and S. Ma, Chem. Commun., 2009, 4572 RSC; (d) W. Kong, C. Fu and S. Ma, Org. Biomol. Chem., 2012, 10, 2164 RSC; (e) W. Kong, Y. Qiu, X. Zhang, C. Fu and S. Ma, Adv. Synth. Catal., 2012, 354, 2339 CrossRef CAS; (f) W. Kong, C. Fu and S. Ma, Chem.–Eur. J., 2011, 17, 13134 CrossRef CAS PubMed; (g) Y. Qiu, D. Ma, C. Fu and S. Ma, Org. Biomol. Chem., 2013, 11, 1666 RSC.
- P. García-García, A. Martínez, M. Sanjuán, M. A. Fernández-Rodríguez and R. Sanz, Org. Lett., 2011, 13, 4970 CrossRef PubMed.
- For selected reports on 1,2-migrations of platinum–carbene intermediates, see: (a) H. Kusama, H. Funami, J. Takaya and N. Iwasawa, Org. Lett., 2004, 6, 605 CrossRef CAS PubMed; (b) H. Kusama, Y. Miyashita, J. Takaya and N. Iwasawa, Org. Lett., 2006, 8, 289 CrossRef CAS PubMed; (c) J. Sun, M. P. Conley, L. Zhang and S. A. Kozmin, J. Am. Chem. Soc., 2006, 128, 9705 CrossRef CAS PubMed; (d) H. Funami, H. Kusama and N. Iwasawa, Angew. Chem., Int. Ed., 2007, 46, 909 CrossRef CAS PubMed; (e) G. Zhang, V. J. Catalano and L. Zhang, J. Am. Chem. Soc., 2007, 129, 11358 CrossRef CAS PubMed; (f) G. Li, X. Huang and L. Zhang, Angew. Chem., Int. Ed., 2008, 47, 346 CrossRef CAS PubMed; (g) D. J. Gorin, N. R. Davis and F. D. Toste, J. Am. Chem. Soc., 2005, 127, 11260 CrossRef CAS PubMed; (h) I. Nakamura, G. B. Bajrachaya, H. Wu, K. Oishi, Y. Mizushima, I. D. Gridnev and Y. Yamamoto, J. Am. Chem. Soc., 2004, 126, 15423 CrossRef CAS PubMed; (i) C. Nieto-Oberhuber, P. M. Muñoz, E. Buñuel, C. Nevado, D. J. Cárdenas and A. M. Echavarren, Angew. Chem., Int. Ed., 2004, 43, 2402 CrossRef CAS PubMed; (j) A. Fürstner, F. Stelzer and H. Szillat, J. Am. Chem. Soc., 2001, 123, 11863 CrossRef; (k) F. Marion, J. Coulomb, C. Courillon, L. Fensterbank and M. Malacria, Org. Lett., 2004, 6, 1509 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures and detailed characterization data for all new compounds. CCDC 944138 and 944139. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c3qo00006k |
| This journal is © the Partner Organisations 2014 |