Investigation of the light-induced electron-transfer-coupled spin transition in a cyanide-bridged [Co2Fe2] complex by X-ray diffraction and absorption measurements†
Yoshihiro
Sekine
a,
Masayuki
Nihei
a,
Reiji
Kumai
b,
Hironori
Nakao
b,
Youichi
Murakami
b and
Hiroki
Oshio
*a
aGraduate School of Pure and Applied Sciences, University of Tsukuba, Tennodai 1-1-1, Tsukuba 305-8571, Japan. E-mail: oshio@chem.tsukuba.ac.jp
bPhoton Factory and Condensed Matter Research Center, Institute of Materials Structures Sciences, High Energy Accelerator Research Organization, 1-1 Oho, Tsukuba 305-0801, Japan
First published on 13th June 2014
Abstract
The light-induced electron-transfer-coupled spin transition (ETCST) behaviour in a cyanide-bridged [Co2Fe2] tetranuclear complex has been investigated in detail, using magnetic susceptibility measurements, single-crystal X-ray structural analyses and X-ray absorption spectroscopy.
Prussian blue (PB) and its analogues (PBAs) have recently attracted great interest resulting from their varied characteristics as functional materials.1 In 1996, a cyanide-bridged CoFe PBA, K0.2Co1.4[Fe(CN)6]·6.9H2O, was reported to show light-induced magnetization from a paramagnetic [ls-CoIII–ls-FeII] phase to a ferrimagnetic [hs-CoII–ls-FeIII] phase (ls = low spin and hs = high spin), where light-induced charge transfer from FeII to CoIII ions caused ls to hs transitions to occur in the Co ions at low temperatures.2 This behavior and its thermally induced equivalent are described as the electron-transfer-coupled spin transition (ETCST).3 Reverse ETCST was also achieved in the CoFe PBA by excitation of the CoII→FeIII charge transfer band. Since the discovery of the light-induced magnetization in CoFe PBAs, numerous heterometallic PBAs have been reported to show light-induced phase transitions,4 and a growing number of cyanide-bridged multinuclear complexes have been intensively investigated to explore the ETCST phenomenon at a molecular level. Light-induced ETCST in a discrete molecule was first reported in an octanuclear [Co4Fe4] complex,5a and in subsequent reports, cyanide-bridged CoFe complexes with a range of nuclearities have been shown to exhibit similar behaviour.5b–l To the best of our knowledge, however, there have been only two reports of structurally characterized light-induced metastable states in the ETCST-active molecules.5e,i Additionally, the electronic structures of the light-induced states in the previous ETCST complexes were only investigated by magnetic susceptibility measurements, and infrared and diffuse reflectance spectroscopy on a bulk sample. X-ray absorption spectroscopy (XAS) is a useful technique to probe the electronic state of transition metal ions. We previously reported a cyanide-bridged tetranuclear complex, [Co2Fe2(CN)6(tp*)2(bpy*)4](PF6)2·2MeOH (1) (tp* = hydrotris(3,5-dimethylpyrazol-1-yl)borate and bpy* = 4,4′-di-tert-butyl-2,2′-bipyridine), which exhibited two-step thermal ETCST and light-induced ETCST behavior.6 Recently, it was also found that 1 exhibits X-ray induced ETCST, a process that was followed by XAS measurements. We report here a detailed investigation of the light-induced ETCST in 1 by means of single crystal X-ray diffraction (XRD) and XAS measurements upon light irradiation.
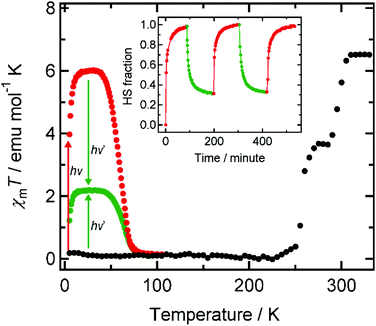
Light irradiation experiments using green and red lasers (λ = 532 and 808 nm) were performed on 1 in the SQUID magnetometer at 5 K, where a ground sample of 1 fixed on a transparent sheet was used to maximize the penetration length of the light. Note that 1 showed FeII→CoIII and CoII→FeIII intervalence charge transfer (IVCT) bands at 770 and 560 nm in its LS and HS states, respectively.6a The ground sample of 1 showed two-step thermal ETCST behavior in the temperature range of 330–5 K as it was cooled prior to light irradiation (Fig. 1). The ETCST behavior is sensitive to the sample preparation and the transition temperatures were slightly lower than those previously reported for a crystalline sample. Upon red light irradiation (808 nm) to the LS state, the χmT values abruptly increased, reaching saturation after 1.5 hours. After deactivating the light source, the χmT values increased gradually with increasing temperature, and a maximum value of 6.00 emu mol−1 K was observed at 34 K, followed by a sudden decrease to almost zero at 85 K. XAS spectra measured at 15 K before and after red light irradiation confirmed that conversion to the metastable HS* state, as a result of light-induced ETCST, was completed (vide infra). Upon green light (532 nm) irradiation to the metastable HS* state, generated from the red light irradiation to the LS state, at 5 K, the χmT values decreased rapidly from 3.97 to 1.22 emu mol−1 K, indicating the reversal of the visible-light-induced ETCST. Increasing the temperature caused an increase in χmT values to a maximum of 2.18 emu mol−1 K at 34 K, at which temperature the reverse conversion ratio (HS*→LS) was estimated to be 64% (= (6.00–2.18)/6.00). The incomplete reverse ETCST was understood as follows: green light (532 nm) irradiation led to a mixture of both (HS*→LS) and (LS→HS*) conversions. Irradiating the sample in the LS state with the green light resulted in spin conversion to the HS* state with a conversion ratio of 36%, meaning that green light irradiation to both the red light-excited HS* and ground LS states allowed the same photostationary state to be accessed. The incomplete phase transition by green light was ascribed to the partial overlap of the broad FeII→CoIII IVCT (εmax = 770 nm) band and the CoII→FeIII IVCT band (εmax = 560 nm).6a That is, the simultaneous green light excitation of the absorption bands corresponding to the two opposing IVCT processes led to the generation of a photostationary state, while red light (808 nm) irradiation excited only the FeII→CoIII IVCT band, and led to complete conversion to the HS* state. It should be noted that the forward (red) and reverse (green) light-induced ETCST could be repeatedly observed when the sample was exposed to alternating red and green light sources (Fig. 1, inset).
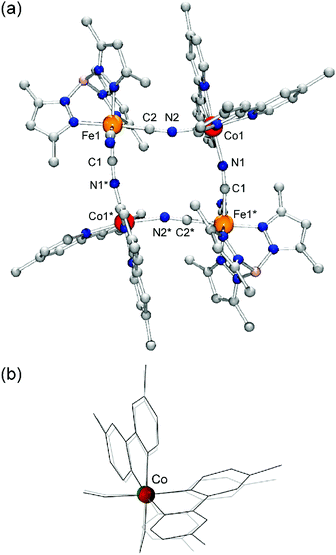
Single crystal X-ray crystallographic analyses of 1 before and after light irradiation at 808 nm were carried out at 24 K using a conventional X-ray diffractometer. Crystallographic data, and selected bond lengths and angles are listed in Tables S1 and S2,† respectively. The molecular structure after visible-light irradiation is shown in Fig. 2(a). 1 crystallized in the monoclinic space group C2/c, and the complex cation has a square-shaped macrocyclic core, in which Co and Fe centers are alternately bridged by cyanide ions. The Fe ions are coordinated by tridentate tp* ligands with facial configuration, and the remaining coordination sites are occupied by three cyanide carbon atoms. The two bidentate bpy* ligands coordinate to the Co ion, while its remaining cis coordination sites are occupied by bridging cyanide ions with [Co–NC–Fe] bridging modes. Prior to visible-light irradiation, as previously reported,7 the average coordination bond lengths around the Co and Fe ions were 1.920(5) Å and 1.960(5) Å, respectively, characteristic of ls CoIII and ls FeII ions.5,6 After the sample was irradiated with 808 nm light for 1.5 hours, the crystallographic parameters changed and the cell volume increased by ca. 7%, suggesting the occurrence of a light-induced phase transition. The average coordination bond lengths after light irradiation changed to 2.12(1) and 1.97(1) Å for Co and Fe ions, respectively, and these values are in good agreement with those (Co: 2.113(4) Å, Fe: 1.964(5) Å) in the thermally generated HS state of 1 at 330 K.6b The distortion of the coordination structure can be quantified by Σ parameters reported by P. Guionneau et al.8 The Σ values for the Co ion before and after visible-light irradiation were estimated as 45.3° and 68.1°, which are typical values for ls CoIII and hs CoII ions, respectively (Fig. 2(b)).5,6 The observed structural changes revealed that the light-induced state is the metastable HS* state generated by light-induced ETCST of the LS state.
Co and Fe K-edge XAS spectra were recorded on a single crystal of 1 to investigate the electronic state changes of the metal ions upon light-induced ETCST. 1 showed absorption edges at 7.729 (ls CoIII) and 7.126 keV (ls FeII) at 15 K,7 where the absorption edge corresponds to the inflection point at the onset of the K-edge absorption peak.9 The XAS spectra of 1 at 15 K agrees well with previously reported spectra of 1 in the LS state.7,10 When a single crystal of 1 in the LS state was irradiated by 808 nm light for 30 minutes at 15 K, the Co absorption edge shifted to lower energy (7.724 keV), while the Fe absorption edge showed a slight shift to higher energy (7.128 keV), suggesting the generation of a metastable HS* state (Fig. 3 and S1†). The resulting Co and Fe K-edge spectra for the HS* state are in accord with those for the thermally generated HS state. The XRD and XAS measurements revealed that the molecular structure and electronic state of the light-induced HS* is almost the same as the thermally generated HS state. Note that the XAS spectra of the light-induced HS* state shifted back to the LS spectra upon heating to 100 K, confirming the occurrence of thermal relaxation (Fig. S2†).
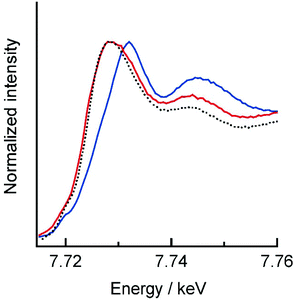 | ||
| Fig. 3 Co K-edge XAS spectra of 1 before (blue) and after (red) 808 nm irradiation at 15 K. The black dashed line represents the thermally generated HS state. | ||
In summary, we investigated the structural and electronic state changes upon visible-light irradiation of 1 by single-crystal XRD and XAS measurements. As a result, both the molecular structure and the electronic state of the light-induced HS* state were coincident with the thermally generated HS state. Furthermore, the reversible magnetic susceptibility changes were demonstrated by alternate irradiation with red and green lasers.
This work was supported by a Grant-in-Aid for Scientific Research on Innovative Areas (“Coordination Programming” Area 2107, no. 21108006) from the Ministry of Education, Culture, Sports, Science and Technology, Japan, and by a Grant-in-Aid for Scientific Research (no. 25248014) from the Japan Society for the Promotion of Science (JSPS), and by the KEK (grant number 2012G036).
Notes and references
- (a) A. Ito, M. Suenaga and K. Ono, J. Chem. Phys., 1968, 48, 3597 CrossRef CAS PubMed; (b) S. Ferlay, T. Mallah, R. Ouahès and M. Verdaguer, Inorg. Chem., 1999, 38, 229 CrossRef CAS; (c) S. Ferlay, T. Mallah, R. Ouahès, P. Veillet and M. Verdaguer, Nature, 1995, 378, 701 CrossRef CAS; (d) T. Mallah, S. Thiébaut, M. Verdaguer and P. Veillet, Science, 1993, 262, 1554 CAS; (e) W. E. Bushmann, J. Ensling, P. Gütlich and J. S. Miller, Chem. – Eur. J., 1999, 5, 3019 Search PubMed; (f) V. Gadet, T. Mallah, I. Castro and M. Verdaguer, J. Am. Chem. Soc., 1992, 114, 9213 CrossRef CAS; (g) S. Ferlay, T. Mallah, R. Ouahès, P. Veillet and M. Verdaguer, Inorg. Chem., 1999, 38, 229 CrossRef CAS; (h) W. R. Entley and G. S. Girolami, Inorg. Chem., 1994, 33, 5165 CrossRef CAS; (i) J. Larionova, R. Clérac, J. Sanchiz, O. Kahn, S. Golhen and L. Ouahab, J. Am. Chem. Soc., 1998, 120, 13088 CrossRef CAS; (j) E. Coronado, M. C. Giménez-López, G. Levchenko, F. M. Romero, V. Garcia-Baonza, A. Milner and M. Paz-Pastemak, J. Am. Chem. Soc., 2005, 127, 4580 CrossRef CAS PubMed; (k) W. Kosaka, K. Nomura, K. Hashimoto and S.-i. Ohkoshi, J. Am. Chem. Soc., 2005, 127, 8590 CrossRef CAS PubMed; (l) S.-i. Ohkoshi, H. Tokoro, T. Matsuda, H. Takahashi, H. Irie and K. Hashimoto, Angew. Chem., Int. Ed., 2007, 46, 3238 CrossRef CAS PubMed.
- O. Sato, T. Iyoda, A. Fujishima and K. Hashimoto, Science, 1996, 272, 704 CAS.
- G. N. Newton, M. Nihei and H. Oshio, Eur. J. Inorg. Chem., 2011, 3031 CrossRef CAS.
- (a) Y. Arimoto, S. Ohkoshi, Z. J. Zhong, H. Seino, Y. Mizobe and K. Hashimoto, J. Am. Chem. Soc., 2003, 125, 9240 CrossRef CAS PubMed; (b) R. Le Bris, C. Mathonière and J.-F. Rétard, Chem. Phys. Lett., 2006, 426, 380 CrossRef CAS PubMed; (c) S. Ohkoshi, S. Ikeda, T. Hozumi, T. Kashiwagi and K. Hashimoto, J. Am. Chem. Soc., 2006, 128, 5320 CrossRef CAS PubMed; (d) S. Ohkoshi, N. Machida, Z. J. Zhong and K. Hashimoto, Synth. Met., 2001, 122, 523 CrossRef CAS; (e) G. Rombaut, M. Verelst, S. Golhen, L. Ouahab, C. Mathonière and O. Kahn, Inorg. Chem., 2001, 40, 1151 CrossRef CAS PubMed; (f) J. M. Herrera, V. Marvaud, M. Verdaguer, J. Marrot, M. Kalisz and C. Mathonière, Angew. Chem., Int. Ed., 2004, 43, 5468 CrossRef CAS PubMed; (g) S. Ohkoshi, H. Tokoro, T. Hozumi, Y. Zhang, K. Hashimoto, C. Mathonière, I. Bord, G. Rombaut, M. Verelst, C. Cartier dit Moulin and F. Villain, J. Am. Chem. Soc., 2006, 128, 270 CrossRef CAS PubMed; (h) H. Tokoro, S. Ohkoshi and K. Hashimoto, Appl. Phys. Lett., 2003, 82, 1245 CrossRef CAS PubMed; (i) H. Tokoro, T. Matsuda, T. Nuida, Y. Moritomo, K. Ohyama, E. D. L. Dangui, K. Boukheddaden and S. Ohkoshi, Chem. Mater., 2008, 20, 423 CrossRef CAS; (j) S. Ohkoshi and T. Tokoro, Acc. Chem. Res., 2012, 45, 1749 CrossRef CAS PubMed.
- (a) D. Li, R. Clérac, O. Roubeau, E. Harté, C. Mathonière, R. L. Bris and S. M. Holmes, J. Am. Chem. Soc., 2008, 130, 252 CrossRef CAS PubMed; (b) T. Liu, D.-P. Dong, S. Kanegawa, S. Kang, O. Sato, Y. Shiota, K. Yoshizawa, S. Hayami, S. Wu, C. He and C.-Y. Duan, Angew. Chem., Int. Ed., 2012, 51, 4367 CrossRef CAS PubMed; (c) Y. Zhang, D. Li, R. Clérac, M. Kalisz, C. Mathonière and S. M. Holmes, Angew. Chem., Int. Ed., 2010, 49, 3752 CrossRef CAS PubMed; (d) J. Mercurol, Y. Li, E. Pardo, O. Risset, M. Seuleiman, H. Rousseliére, R. Lescouëzec and M. Julve, Chem. Commun., 2010, 46, 8995 RSC; (e) A. Mondal, Y. Li, M. Seuleiman, M. Julve, L. Toupet, M. B.-L. Cointe and R. Lescouëzec, J. Am. Chem. Soc., 2013, 135, 1653 CrossRef CAS PubMed; (f) D.-P. Dong, T. Liu, S. Kanegawa, S. Kang, O. Sato, C. He and C.-Y. Duan, Angew. Chem., Int. Ed., 2012, 51, 5119 CrossRef CAS PubMed; (g) L. Cao, J. Tao, Q. Gao, T. Liu, Z. Xia and D. Li, Chem. Commun., 2014, 50, 1665 RSC; (h) K. E. Funck, A. V. Prosvirin, C. Mathonière, R. Clérac and K. R. Dunbar, Inorg. Chem., 2011, 50, 2782 CrossRef CAS PubMed; (i) M. Nihei, Y. Okamoto, Y. Sekine, N. Hoshino, T. Shiga, I.-P. Liu and H. Oshio, Angew. Chem., Int. Ed., 2012, 51, 6361 CrossRef CAS PubMed; (j) K. Mitsumoto, E. Oshiro, H. Nishikawa, T. Shiga, Y. Yamamura, K. Saito and H. Oshio, Chem. – Eur. J., 2011, 17, 9612 CrossRef CAS PubMed; (k) N. Hoshino, F. Iijima, G. N. Newton, N. Yoshida, T. Shiga, H. Nojiri, A. Nakao, R. Kumai, Y. Murakami and H. Oshio, Nat. Chem., 2012, 4, 921 CrossRef CAS PubMed; (l) Y. Sekine, M. Nihei and H. Oshio, Chem. Lett, in press, DOI:10.1246/cl.140248.
- (a) M. Nihei, Y. Sekine, N. Suganami, K. Nakazawa, A. Nakao, H. Nakao, Y. Murakami and H. Oshio, J. Am. Chem. Soc., 2011, 133, 3592 CrossRef CAS PubMed; (b) M. Nihei, Y. Sekine, N. Suganami and H. Oshio, Chem. Lett., 2010, 39, 978 CrossRef CAS.
- Y. Sekine, M. Nihei, R. Kumai, H. Nakao, Y. Murakami and H. Oshio, Chem. Commun., 2014, 50, 4050 RSC.
- P. Guionneau, M. Marchivie, G. Bravic, J.-F. Letard and D. Chasseau, J. Mater. Chem., 2002, 12, 2546 RSC.
- B. K. Teo, EXAFS: Basic Principles and Data Analysis, Springer-Verlag, Berlin, 1986 Search PubMed.
- It should be noted that the relative small energy shift of Fe K edges associated with the thermal and light-induced ETCST were attributed to the small structural changes between the ls FeIII and the ls FeII ions.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental and crystallographic details and XAS spectra. CCDC 992600 (after light irradiation). For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c4qi00074a |
| This journal is © the Partner Organisations 2014 |