Magnetocaloric effect and slow magnetic relaxation in two dense (3,12)-connected lanthanide complexes†
Song-De
Han
,
Xiao-Hong
Miao
,
Sui-Jun
Liu
and
Xian-He
Bu
*
Department of Chemistry, TKL of Metal- and Molecule-Based Material Chemistry and Collaborative Innovation Center of Chemical Science and Engineering (Tianjin), Nankai University, Tianjin 300071, P.R. China. E-mail: buxh@nankai.edu.cn; Fax: +86-22-23502458
First published on 24th June 2014
Abstract
Two (3,12)-connected complexes with distorted cubic [Ln4O4] (Ln = Gd (1), Dy (2)) building units were synthesized. The magnetic studies reveal that 1 features a large magnetocaloric effect with −ΔSmaxm = 51.29 J kg−1 K−1, and 2 displays slow magnetic relaxation behavior.
The magnetocaloric effect (MCE), defined as the changes of the isothermal magnetic entropy (ΔSm) and adiabatic temperature (ΔTad) of a magnetic material induced by a change in the applied magnetic field (ΔH), was first discovered in 1881 by Warburg.1 Magnetic refrigeration, based on the MCE, has received much interest recently due to the possibility of replacing the expensive and increasingly rare He-3 in ultralow-temperature refrigeration.2 Since observations of the MCE in Mn12 and Fe8 molecular clusters opened the door to molecular magnetic cryogenic materials, this fascinating field has attracted much attention from chemists and has been rapidly expanding, stemming from not only its highly-efficient, environmentally friendly, and energy-saving superiority, but also the possibilities for synthetic tunability and functional tailorability.3–8
To design molecular magnetic coolers with remarkable MCE, GdIII with seven unpaired 4f electrons is a promising candidate as the intrinsic nature of GdIII fulfils the requirements for improving the MCE well, such as a large spin ground state S, negligible magnetic anisotropy (Dion = 0) and low-lying excited spin states.4 Furthermore, due to the efficient shielding of the 4f orbitals of the GdIII ion, the magnetic interactions of GdIII–GdIII/3d–GdIII are usually anticipated to be weak, which is also helpful to promote the MCE. Hence, the assembly of GdIII/3d–GdIII with light or polydentate ligands is a feasible strategy to achieve the aforementioned target, which has been well corroborated in recent publications.5,6 To our knowledge, most of the reported Gd-based complexes with significant MCE are discrete [Gdn]4a,5 and [3d–Gd]6 (3d = Cr, Mn, Fe, Co, Ni, Cu) clusters and a few cases are low dimensional Gd-based coordination polymers.4a,7 Research into the MCE in 3D coordination polymers is still rare.8 The potential advantages of the 3D framework are not only that the adjacent metal ions/metal clusters share the bridging ligands, which can enhance the magnetic density and result in a large MCE, but also that they have relatively higher thermal and/or solvent stabilities than discrete molecular clusters, which provides a solid foundation for future applications.3e,f,8b,c However, to date, most of the reported Gd-based complexes with significant MCE from 0D discrete clusters to 3D frameworks are constructed via organic ligands,5,6 and cases fabricated by inorganic ligands are very rare.8c,i In fact, some inorganic ligands like sulfate, phosphate, carbonate are also polydentate. For example, the tetradentate sulfate with rich coordination modes preferably bonds to lanthanide ions, has been widely utilized to synthesize metal–organic frameworks (MOFs).9 Moreover, weak exchange interactions and relatively high density may be expected in Gd-containing complexes bridged by sulfate.9b,10 Both of these characteristics are helpful in enhancing the performance in MCE, which was well demonstrated by the early studied molecular magnetic cooler [Gd2(SO4)3·8H2O].11
On the other hand, Dy clusters are being intensively explored in single-molecule magnets (SMMs) due to the large single-ion magnetic anisotropy of DyIII. Hitherto, various [Dyn] clusters with slow magnetic relaxation behavior have been reported, including Dy2,12a Dy3,12b Dy4,12c Dy5,12d Dy6,12e Dy7,12f Dy8,12g Dy9,12h Dy10,12i Dy11,12j Dy12,12k Dy14,12l Dy24.5b However, observations of such behavior in 2D or 3D complexes utilizing Dy clusters as nodes remain scarce.8c,13 Therefore, it is interesting and worthwhile to investigate the magnetic properties of high dimensional complexes based on Dy clusters as nodes.
Herein, we report the synthesis, structures, and magnetic properties of two (3,12)-connected sulfate-based lanthanide complexes [Ln4(SO4)4(μ3-OH)4(H2O)n] (Ln = Gd, n = 4 for 1, and Ln = Dy, n = 3 for 2). Magnetic measurements demonstrate that complex 1 displays a magnetic entropy change −ΔSmaxm = 51.29 J kg−1 K−1, being the third largest value to date, and simultaneously presents high solvent stabilities. Complex 2 exhibits slow magnetic relaxation behavior at low temperature, which is rare in 2D or 3D complexes based on Dy clusters as nodes. Colorless crystals of 1 and 2 were obtained by the solvothermal reaction of Hbms·HCl (Hbms = (1H-benzimidazol-2-yl)methanethiol), and Ln(NO3)3·6H2O in a CH3OH–H2O mixed solvent at 140 °C. It is notable that the sulfate is generated in situ due to the oxidation of thiol under acid conditions in the presence of NO3−. The in situ transformation of thiol to sulfate has been documented in a recent publication.14 The SO42− in 1 and 2 can also be unequivocally demonstrated by the IR characteristic peaks at about 1100 cm−1 (Fig. S12†).
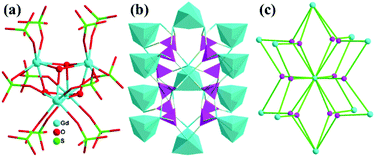
Two similar complexes (Ln = Y, Er) with differences only in the terminal ligands on the GdIII ions for 1 have been previously reported.15 Therefore, the structure of 1 is discussed briefly here. Complex 1 crystallizes in the orthorhombic space group P212121 and contains hydroxyl bridged tetranuclear units [Gd4(μ3-OH)4(H2O)4]8+ ([Gd4], for short), which are further connected via SO42− ligands to generate a 3D framework. Each GdIII connects three neighboring GdIII ions with four μ3-OH− bridging ligands, forming a distorted cubic [Gd4] unit, in which four GdIII ions are located at the corners of the tetrahedron. Each SO42− bridges three [Gd4] units, and each [Gd4] unit links twelve SO42− groups (Fig. 1a and 1b). Thus the SO42− and [Gd4] cluster can be considered as 3-connected and 12-connected nodes, respectively, giving rise to a unique (3,12)-connected topological network with the point (Schläfli) symbol of (420·628·818)(43)4 calculated by TOPOS (Fig. 1c).16 To our knowledge, several (3,12)-connected frameworks have been reported, while only four examples are based on multinuclear lanthanide clusters.8c,14,17
Both 1 and 2 are stable in air, confirmed by their simulated and experimental PXRD (powder X-ray diffraction) patterns (Fig. S1†). Importantly, the simulated PXRD pattern of 1 from single crystal data reaches good agreement with the corresponding experimental ones after 1 is soaked in common solvents, such as H2O, CH3OH, CH3CH2OH, CH3CN, CH2Cl2, acetone, DMF, tetrahydrofuran, and cyclohexane solution for 48 hours (Fig. S1†), which notably shows that 1 possesses excellent and extensive solvent stability.
The phase purity of complexes 1 and 2 was confirmed by PXRD patterns (Fig. S1 and S2†). Variable-temperature magnetic susceptibility measurements were investigated on polycrystalline samples of complexes 1 and 2 with an applied dc field of 1000 Oe (Fig. S3†). The observed χMT products of 31.61 and 55.73 cm3 mol−1 K at 300 K for 1 and 2 are in good agreement with the theoretical values for the unit of four non-interacting GdIII (31.50 cm3 K mol−1, 8S7/2, g = 2) in 1 and four isolated DyIII (56.67 cm3 K mol−1, 6H15/2, g = 4/3) in 2. Upon cooling, the χMT value of 1 stays essentially constant until approximately 25 K, while the χMT value of 2 gradually decreases from 300 K to 50 K, followed by an obvious decrease to the minimum value of 19.74 cm3 K mol−1 for 1 and 38.88 cm3 K mol−1 for 2 at 2 K. The decline of the curve for 1 indicates that antiferromagnetic coupling exists between adjacent GdIII ions, which is further corroborated by the negative Weiss constant θ = −1.57 K from Curie–Weiss fitting (Fig. S4†).
Magnetization measurements were investigated in the range 0–7 T at 2–10 K for 1 and 0–7 T at 2 K for 2 (Fig. S5 and S6†). The plots of M versus H display a steady increase with the increasing field. For 1, M reaches a value of 28.30Nβ at 2 K and 7 T, which agrees with the theoretical value of 28Nβ for four GdIII (g = 2, S = 7/2). For 2, the M value is 25.20Nβ at 2 K and 7 T, far from the theoretical saturated value of 40Nβ for four DyIII (g = 4/3, J = 15/2). The magnetic unsaturation even at 7 T may be attributed to the magnetic anisotropy and/or low-lying excited states of DyIII, which is supported by the non-superposition of magnetization curves at different temperatures (Fig. S7†). The beautiful superposition of the field-cooled (FC) curve and the zero-field-cooled (ZFC) curve preclude the existence of long-range magnetic ordering above 2 K in 1 (Fig. S8†).
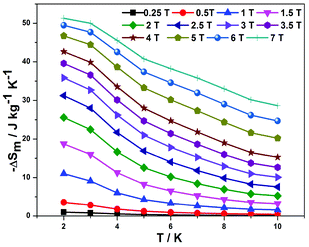
The weak magnetic interactions and large metal/ligand mass ratio make complex 1 a promising candidate for low-temperature magnetic cooling, as magnetic entropy change ΔSm, a key parameter in evaluating the MCE, can be derived by applying the Maxwell equation ΔSm(T)ΔH = ∫[∂M(T,H)/∂T]HdH to the experimentally obtained magnetization data.5–8 The entropy changes at various magnetic fields and temperatures are summarized in Fig. 2, with an impressive −ΔSmaxm of 51.29 J kg−1 K−1 for T = 2 K and ΔH = 7 T. The value of −ΔSmaxm is smaller than the value of 59.96 J kg−1 K−1 for four uncoupled GdIII (judged by 4R![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ln(2S + 1), where R is the gas constant and S is the spin state). The gap between the experimental data and the theoretical value mainly originates from the intracluster antiferromagnetic interactions in 1. Among reported molecule-based magnetic cryogens, magnetic entropy changes −ΔSmaxm above 40.0 J kg−1 K−1 are limited, as listed in Table S1.† It is notable that only three cases with magnetic entropy change −ΔSmaxm above 50.0 J kg−1 K−1 have been reported. It is still very competitive when considered from the volumetric aspect with −ΔSmaxm = 198.85 mJ cm−3 K−1, being among the highest throughout the GdIII-containing complexes (Table S1†). Indeed, evaluating molecule-based magnetocaloric materials from a volumetric aspect is more meaningful for practical application.18 The large MCE per unit mass and/or per unit volume of 1 is primarily ascribed to the high magnetic density and the small linker between the clusters. Furthermore, the −ΔSm per unit mass reaches a value of 42.63 J kg−1 K−1 for T = 2 K and ΔH = 40 kG, which is larger than for most reported GdIII-based complexes.4–7,8a–g
ln(2S + 1), where R is the gas constant and S is the spin state). The gap between the experimental data and the theoretical value mainly originates from the intracluster antiferromagnetic interactions in 1. Among reported molecule-based magnetic cryogens, magnetic entropy changes −ΔSmaxm above 40.0 J kg−1 K−1 are limited, as listed in Table S1.† It is notable that only three cases with magnetic entropy change −ΔSmaxm above 50.0 J kg−1 K−1 have been reported. It is still very competitive when considered from the volumetric aspect with −ΔSmaxm = 198.85 mJ cm−3 K−1, being among the highest throughout the GdIII-containing complexes (Table S1†). Indeed, evaluating molecule-based magnetocaloric materials from a volumetric aspect is more meaningful for practical application.18 The large MCE per unit mass and/or per unit volume of 1 is primarily ascribed to the high magnetic density and the small linker between the clusters. Furthermore, the −ΔSm per unit mass reaches a value of 42.63 J kg−1 K−1 for T = 2 K and ΔH = 40 kG, which is larger than for most reported GdIII-based complexes.4–7,8a–g
To further explore the magnetic dynamics of 2, the frequency and temperature dependencies of the alternating current (AC) susceptibilities were collected under a zero direct current (DC) field and a 3 Oe AC magnetic field (Fig. 3 and Fig. S9†). The out-of-phase constituent of the AC susceptibilities exhibits frequency dependent signals below 15 K, suggesting the slow magnetic relaxation behaviour of 2, which might be an indication of SMM behaviour. However, similar to some reported Dy-based complexes,19 the peaks of the out-of-phase signals were not observed in the technically available temperature range due to fast quantum tunneling. To reduce the quantum tunneling effect, a proper DC field needs to be exerted. The optimal field was found from field-dependent AC susceptibility measurements (0–5 kOe). As shown in Fig. S10,† the peak of χ′′M appeared at ca. 800 Oe. Thus, an 800 Oe DC field was exerted. However, the peak of χ′′M still could not be observed because the strong quantum tunneling effect was not effectively suppressed (Fig. S11†).
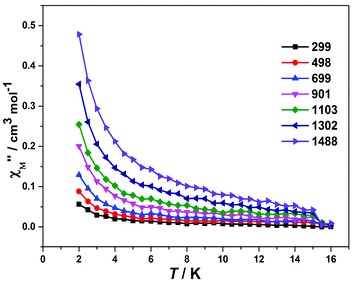 | ||
| Fig. 3 Frequency dependence of the out-of-phase (χ′′) ac susceptibility components for 2 at zero dc field. | ||
Conclusions
In conclusion, two (3,12)-connected complexes based on cubic [Ln4O4] clusters bridged by sulfate were structurally and magnetically characterized. The significant MCE of 1 and the slow magnetic relaxation behaviour of 2 were observed. More importantly, 1 shows a large −ΔSm value of 51.29 J kg−1 K−1, being among the highest values in reported molecular magnetic cooling Gd-based compounds known to date. The high MCE makes 1 a good candidate for the application of cryogenic refrigeration. The successful synthesis of 1 not only enriches the existing field of molecular magnetic coolers, but also confirms the potential of developing other outstanding complexes with remarkable MCE via the assembly of suitable inorganic ligands and metal ions.Acknowledgements
This work was supported by the 973 Program of China (Grants 2014CB845600 and 2012CB821700) and the NSF of China (Grants 21031002 and 21290171).Notes and references
- For examples, see: (a) E. Warburg, Ann. Phys., 1881, 249, 141 CrossRef; (b) E. Warburg, Ann. Phys. Chem., 1881, 13, 141 CrossRef; (c) P. Debye, Ann. Phys., 1926, 386, 1154 CrossRef.
- (a) K. A. Gschneidner Jr. and V. K. Pecharsky, Int. J. Refrig., 2008, 31, 945 CrossRef PubMed; (b) M. Evangelisti and E. K. Brechin, Dalton Trans., 2010, 39, 4672 RSC; (c) D. Gatteschi and R. Sessoli, Angew. Chem., Int. Ed., 2003, 42, 268 CrossRef CAS PubMed.
- (a) M. Evangelisti, F. Luis, L. J. de Jongh and M. Affronte, J. Mater. Chem., 2006, 16, 2534 RSC; (b) T. N. Hooper, J. Schnack, S. Piligkos, M. Evangelisti and E. K. Brechin, Angew. Chem., Int. Ed., 2012, 51, 4633 CrossRef CAS PubMed; (c) G. Karotsis, M. Evangelisti, S. J. Dalgarno and E. K. Brechin, Angew. Chem., Int. Ed., 2009, 48, 9928 CrossRef CAS PubMed; (d) Y. Z. Zheng, M. Evangelisti, F. Tuna and R. E. P. Winpenny, J. Am. Chem. Soc., 2012, 134, 1057 CrossRef CAS PubMed; (e) J. W. Sharples and D. Collison, Polyhedron, 2013, 54, 91 CrossRef CAS PubMed; (f) Y. Z. Zheng, G. J. Zhou, Z. Zheng and R. E. P. Winpenny, Chem. Soc. Rev., 2014, 43, 1462 RSC.
- (a) F. S. Guo, J. D. Leng, J. L. Liu, Z. S. Meng and M. L. Tong, Inorg. Chem., 2012, 51, 405 CrossRef CAS PubMed; (b) M. Evangelisti, O. Roubeau, E. Palacios, A. Camon, T. N. Hooper, E. K. Brechin and J. J. Alonso, Angew. Chem., Int. Ed., 2011, 50, 6606 CrossRef CAS PubMed.
- (a) J. W. Sharples, Y. Z. Zheng, F. Tuna, E. J. McInnes and D. Collison, Chem. Commun., 2011, 47, 7650 RSC; (b) L. X. Chang, G. Xiong, L. Wang, P. Cheng and B. Zhao, Chem. Commun., 2013, 49, 1055 RSC; (c) S. J. Liu, J. P. Zhao, J. Tao, J. M. Jia, S. D. Han, Y. Li, Y. C. Chen and X. H. Bu, Inorg. Chem., 2013, 52, 9163 CrossRef CAS PubMed; (d) F. S. Guo, Y. C. Chen, L. L. Mao, W. Q. Lin, J. D. Leng, R. Tarasenko, M. Orendáč, J. Prokleška, V. Sechovský and M. L. Tong, Chem. – Eur. J., 2013, 19, 14876 CrossRef CAS PubMed.
- (a) T. Birk, K. S. Pedersen, C. A. Thuesen, T. Weyhermuller, M. Schau-Magnussen, S. Piligkos, H. Weihe, S. Mossin, M. Evangelisti and J. Bendix, Inorg. Chem., 2012, 51, 5435 CrossRef CAS PubMed; (b) Y. Z. Zheng, E. M. Pineda, M. Helliwell and R. E. P. Winpenny, Chem. – Eur. J., 2012, 18, 4161 CrossRef CAS PubMed; (c) E. Cremades, S. Gomez-Coca, D. Aravena, S. Alvarez and E. Ruiz, J. Am. Chem. Soc., 2012, 134, 10532 CrossRef CAS PubMed; (d) J. B. Peng, Q. C. Zhang, Y. Z. Zheng, X. J. Kong, Y. P. Ren, L. S. Long, R. B. Huang, L. S. Zheng and Z. P. Zheng, J. Am. Chem. Soc., 2012, 134, 3314 CrossRef CAS PubMed; (e) J. D. Leng, J. L. Liu and M. L. Tong, Chem. Commun., 2012, 48, 5286 RSC.
- (a) G. Lorusso, M. A. Palacios, G. S. Nichol, E. K. Brechin, O. Roubeau and M. Evangelisti, Chem. Commun., 2012, 48, 7592 RSC; (b) S. Biswas, A. Adhikary, S. Goswami and S. Konar, Dalton Trans., 2013, 42, 13331 RSC; (c) M. Wu, F. Jiang, X. Kong, D. Yuan, L. Long, S. A. Al-Thabaiti and M. Hong, Chem. Sci., 2013, 4, 3104 RSC; (d) S. J. Liu, C. C. Xie, J. M. Jia, J. P. Zhao, S. D. Han, Y. Cui, Y. Li and X. H. Bu, Chem. – Asian J., 2014, 9, 1116 CrossRef CAS PubMed.
- (a) L. Sedláková, J. Hanko, A. Orendáčová, M. Orendáč, C. L. Zhou, W. H. Zhu, B. W. Wang, Z. M. Wang and S. Gao, J. Alloys Compd., 2009, 487, 425 CrossRef PubMed; (b) P. F. Shi, Y. Z. Zheng, X. Q. Zhao, G. Xiong, B. Zhao, F. F. Wan and P. Cheng, Chem. – Eur. J., 2012, 18, 15086 CrossRef CAS PubMed; (c) Y. L. Hou, G. Xiong, P. F. Shi, R. R. Cheng, J. Z. Cui and B. Zhao, Chem. Commun., 2013, 49, 6066 RSC; (d) R. Sibille, T. Mazet, B. Malaman and M. Francois, Chem. – Eur. J., 2012, 18, 12970 CrossRef CAS PubMed; (e) J. M. Jia, S. J. Liu, Y. Cui, S. D. Han, T. L. Hu and X. H. Bu, Cryst. Growth Des., 2013, 13, 4631 CrossRef CAS; (f) F. S. Guo, Y. C. Chen, J. L. Liu, J. D. Leng, Z. S. Meng, P. Vrábel, M. Orendáč and M. L. Tong, Chem. Commun., 2012, 48, 12219 RSC; (g) Y. C. Chen, F. S. Guo, Y. Z. Zheng, J. L. Liu, J. D. Leng, R. Tarasenko, M. Orendáč, J. Prokleška, V. Sechovský and M. L. Tong, Chem. – Eur. J., 2013, 19, 13504 CrossRef CAS PubMed; (h) G. Lorusso, J. W. Sharples, E. Palacios, O. Roubeau, E. K. Brechin, R. Sessoli, A. Rossin, F. Tuna, E. J. L. McInnes, D. Collison and M. Evangelisti, Adv. Mater., 2013, 25, 4653 CrossRef CAS PubMed; (i) Y.-C. Chen, L. Qin, Z.-S. Meng, D. Yang, C. Wu, Z. Fu, Y.-Z. Zheng, J.-L. Liu, R. Tarasenko, M. Orendáč, J. Prokleska, V. Sechovský and M.-L. Tong, J. Mater. Chem. A, 2014, 2, 9851 RSC.
- (a) Y. Q. Sun, J. Zhang and G. Y. Yang, Chem. Commun., 2006, 1947 RSC; (b) Y. Xing, Z. Shi, G. Li and W. Pang, Dalton Trans., 2003, 940 RSC.
- H. Xiang, W.-G. Lu, W.-X. Zhang and L. Jiang, Dalton Trans., 2013, 42, 867 RSC.
- (a) W. F. Giauque and D. P. MacDougall, Phys. Rev., 1933, 43, 0768 CrossRef CAS; (b) W. F. Giauque and D. P. MacDougall, J. Am. Chem. Soc., 1935, 57, 1175 CrossRef CAS.
- (a) Y. N. Guo, G. F. Xu, W. Wernsdorfer, L. Ungur, Y. Guo, J. Tang, H. J. Zhang, L. F. Chibotaru and A. K. Powell, J. Am. Chem. Soc., 2011, 133, 11948 CrossRef CAS PubMed; (b) F. S. Guo, J. L. Liu, J. D. Leng, Z. S. Meng, Z. J. Lin, M. L. Tong, S. Gao, L. Ungur and L. F. Chibotaru, Chem. – Eur. J., 2011, 17, 2458 CrossRef CAS PubMed; (c) P. H. Lin, T. J. Burchell, L. Ungur, L. F. Chibotaru, W. Wernsdorfer and M. Murugesu, Angew. Chem., Int. Ed., 2009, 48, 9489 CrossRef CAS PubMed; (d) M. T. Gamer, Y. Lan, P. W. Roesky, A. K. Powell and R. Clerac, Inorg. Chem., 2008, 47, 6581 CrossRef CAS PubMed; (e) H. Tian, M. Wang, L. Zhao, Y. N. Guo, Y. Guo, J. Tang and Z. Liu, Chem. – Eur. J., 2012, 18, 442 CrossRef CAS PubMed; (f) A. B. Canaj, D. I. Tzimopoulos, A. Philippidis, G. E. Kostakis and C. J. Milios, Inorg. Chem., 2012, 51, 7451 CrossRef CAS PubMed; (g) P. P. Yang, X. F. Gao, H. B. Song, S. Zhang, X. L. Mei, L. C. Li and D. Z. Liao, Inorg. Chem., 2011, 50, 720 CrossRef CAS PubMed; (h) X. Xu, L. Zhao, G. F. Xu, Y. N. Guo, J. K. Tang and Z. L. Liu, Dalton Trans., 2011, 40, 6440 RSC; (i) H. S. Ke, G. F. Xu, L. Zhao, J. K. Tang, X. Y. Zhang and H. J. Zhang, Chem. – Eur. J., 2009, 15, 10335 CrossRef CAS PubMed; (j) Y. L. Miao, J. L. Liu, J. Y. Li, J. D. Leng, Y. C. Ou and M. L. Tong, Dalton Trans., 2011, 40, 10229 RSC; (k) Y. L. Miao, J. L. Liu, J. D. Leng, Z. J. Lin and M. L. Tong, CrystEngComm, 2011, 13, 3345 RSC; (l) X. L. Li, L. F. He, X. L. Feng, Y. Song, M. Hu, L. F. Han, X. J. Zheng, Z. H. Zhang and S. M. Fang, CrystEngComm, 2011, 13, 3643 RSC.
- B. Q. Ma, D. S. Zhang, S. Gao, T. Z. Jin, C. H. Yan and G. S. Xu, Angew. Chem., Int. Ed., 2000, 39, 3644 CrossRef CAS.
- J. J. Guo, W. Wang, Y.-D. Zhang, L. Yang, S.-H. Zhang and X.-Q. Zhang, J. Coord. Chem., 2013, 66, 1467 CrossRef CAS.
- W. H. Wang, H. R. Tian, Z. C. Zhou, Y. L. Feng and J. W. Cheng, Cryst. Growth Des., 2012, 12, 2567 CAS.
- V. A. Blatov, TOPOS, A multipurpose Crystallochemical Analysis with the Program Package, Samara State University, Russia, 2004 Search PubMed.
- (a) Y. Q. Sun, H. X. Mei, H. H. Zhang, Y. P. Chen and R. Q. Sun, J. Cluster Sci., 2011, 22, 279 CrossRef CAS; (b) X. Li, H. L. Sun, X. S. Wu, X. Qiu and M. Du, Inorg. Chem., 2010, 49, 1865 CrossRef CAS PubMed.
- (a) K. A. Gschneidner Jr., V. K. Pecharsky and A. O. Tsokol, Rep. Prog. Phys., 2005, 68, 1479 CrossRef; (b) K. A. Gschneidner Jr. and V. K. Pecharsky, Annu. Rev. Mater. Sci., 2000, 30, 387 CrossRef.
- For examples, see: (a) H. S. Ke, G. F. Xu, L. Zhao, J. K. Tang, X. Y. Zhang and H. J. Zhang, Chem. – Eur. J., 2009, 15, 10335 CrossRef CAS PubMed; (b) Y. Z. Zheng, Y. Lan, C. E. Anson and A. K. Powell, Inorg. Chem., 2008, 47, 10813 CrossRef CAS PubMed; (c) M. T. Gamer, Y. Lan, P. W. Roesky, A. K. Powell and R. Clerac, Inorg. Chem., 2008, 47, 581 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 981569 and 981570. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c4qi00073k |
| This journal is © the Partner Organisations 2014 |